MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1
- PMID: 18227515
- PMCID: PMC2234176
- DOI: 10.1073/pnas.0707493105
MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1
Abstract
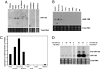
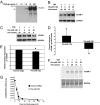
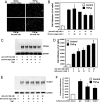
Adhesion molecules expressed by activated endothelial cells play a key role in regulating leukocyte trafficking to sites of inflammation. Resting endothelial cells normally do not express adhesion molecules, but cytokines activate endothelial cells to express adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1), which mediate leukocyte adherence to endothelial cells. We now show that endothelial cells express microRNA 126 (miR-126), which inhibits VCAM-1 expression. Transfection of endothelial cells with an oligonucleotide that decreases miR-126 permits an increase in TNF-alpha-stimulated VCAM-1 expression. Conversely, overexpression of the precursor to miR-126 increases miR-126 levels and decreases VCAM-1 expression. Additionally, decreasing endogenous miR-126 levels increases leukocyte adherence to endothelial cells. These data suggest that microRNA can regulate adhesion molecule expression and may provide additional control of vascular inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells.J Immunol. 1996 Apr 1;156(7):2558-65. J Immunol. 1996. PMID: 8786319
-
Shear-sensitive microRNA-34a modulates flow-dependent regulation of endothelial inflammation.J Cell Sci. 2015 Jan 1;128(1):70-80. doi: 10.1242/jcs.154252. Epub 2014 Nov 13. J Cell Sci. 2015. PMID: 25395581
-
A novel thiazolidinedione MCC-555 down-regulates tumor necrosis factor-alpha-induced expression of vascular cell adhesion molecule-1 in vascular endothelial cells.Atherosclerosis. 2005 Sep;182(1):71-7. doi: 10.1016/j.atherosclerosis.2005.02.004. Epub 2005 Mar 4. Atherosclerosis. 2005. PMID: 16115476
-
MicroRNAs as pharmacological targets in endothelial cell function and dysfunction.Pharmacol Res. 2013 Sep;75:15-27. doi: 10.1016/j.phrs.2013.04.002. Epub 2013 Apr 18. Pharmacol Res. 2013. PMID: 23603154 Free PMC article. Review.
-
Post-transcriptional gene regulation by RNA-binding proteins in vascular endothelial dysfunction.Sci China Life Sci. 2014 Aug;57(8):836-44. doi: 10.1007/s11427-014-4703-5. Epub 2014 Aug 8. Sci China Life Sci. 2014. PMID: 25104457 Free PMC article. Review.
Cited by
-
Exploring the diagnostic potential of miRNA signatures in the Fabry disease serum: A comparative study of automated and manual sample isolations.PLoS One. 2024 Oct 28;19(10):e0301733. doi: 10.1371/journal.pone.0301733. eCollection 2024. PLoS One. 2024. PMID: 39466827 Free PMC article.
-
The Role of MicroRNA in the Pathophysiology and Diagnosis of Viral Myocarditis.Int J Mol Sci. 2024 Oct 11;25(20):10933. doi: 10.3390/ijms252010933. Int J Mol Sci. 2024. PMID: 39456716 Free PMC article. Review.
-
MicroRNA signatures in the pathogenesis and therapy of inflammatory bowel disease.Clin Exp Med. 2024 Sep 11;24(1):217. doi: 10.1007/s10238-024-01476-z. Clin Exp Med. 2024. PMID: 39259390 Free PMC article. Review.
-
Epigenetics and Control of Tumor Angiogenesis in Melanoma: An Update with Therapeutic Implications.Cancers (Basel). 2024 Aug 14;16(16):2843. doi: 10.3390/cancers16162843. Cancers (Basel). 2024. PMID: 39199614 Free PMC article. Review.
-
miRNAs as potential biomarkers for subclinical atherosclerosis in Sjögren's disease.RMD Open. 2024 Aug 22;10(3):e004434. doi: 10.1136/rmdopen-2024-004434. RMD Open. 2024. PMID: 39179256 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

