IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland
- PMID: 18060036
- PMCID: PMC2096439
- DOI: 10.1172/JCI32533
IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland
Abstract
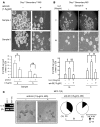
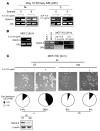
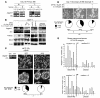
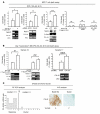
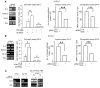
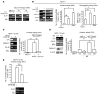
High serum levels of IL-6 correlate with poor outcome in breast cancer patients. However, no data are available on the relationship between IL-6 and mammary stem/progenitor cells, which may fuel the genesis of breast cancer in vivo. Herein, we address this issue in the MCF-7 breast cancer cell line and in primary human mammospheres (MS), multicellular structures enriched in stem/progenitor cells of the mammary gland. MS from node invasive breast carcinoma tissues expressed IL-6 mRNA at higher levels than did MS from matched non-neoplastic mammary glands. In addition, IL-6 mRNA was detected only in basal-like breast carcinoma tissues, an aggressive breast carcinoma variant showing stem cell features. IL-6 treatment triggered Notch-3-dependent upregulation of the Notch ligand Jagged-1 and promotion of MS and MCF-7-derived spheroid growth. Moreover, IL-6 induced Notch-3-dependent upregulation of the carbonic anhydrase IX gene and promoted a hypoxia-resistant/invasive phenotype in MCF-7 cells and MS. Finally, autocrine IL-6 signaling relied upon Notch-3 activity to sustain the aggressive features of MCF-7-derived hypoxia-selected cells. In conclusion, these data support the hypothesis that IL-6 induces malignant features in Notch-3-expressing stem/progenitor cells from human ductal breast carcinoma and normal mammary gland.
Figures



Comment in
-
IL-6 involvement in epithelial cancers.J Clin Invest. 2007 Dec;117(12):3660-3. doi: 10.1172/JCI34237. J Clin Invest. 2007. PMID: 18060028 Free PMC article.
Similar articles
-
IL-6 involvement in epithelial cancers.J Clin Invest. 2007 Dec;117(12):3660-3. doi: 10.1172/JCI34237. J Clin Invest. 2007. PMID: 18060028 Free PMC article.
-
Interleukin-6 and pro inflammatory status in the breast tumor microenvironment.World J Surg Oncol. 2015 Mar 28;13:129. doi: 10.1186/s12957-015-0529-2. World J Surg Oncol. 2015. PMID: 25881039 Free PMC article.
-
The basal-like breast carcinoma phenotype is regulated by SLUG gene expression.J Pathol. 2008 Jan;214(1):25-37. doi: 10.1002/path.2254. J Pathol. 2008. PMID: 17973239
-
Mammary stem cells and breast cancer--role of Notch signalling.Stem Cell Rev. 2007 Jun;3(2):169-75. doi: 10.1007/s12015-007-0023-5. Stem Cell Rev. 2007. PMID: 17873349 Review.
-
Mammary Development and Breast Cancer: a Notch Perspective.J Mammary Gland Biol Neoplasia. 2021 Sep;26(3):309-320. doi: 10.1007/s10911-021-09496-1. Epub 2021 Aug 10. J Mammary Gland Biol Neoplasia. 2021. PMID: 34374886 Free PMC article. Review.
Cited by
-
Synergistic effect of the γ-secretase inhibitor PF-03084014 and docetaxel in breast cancer models.Stem Cells Transl Med. 2013 Mar;2(3):233-42. doi: 10.5966/sctm.2012-0096. Epub 2013 Feb 13. Stem Cells Transl Med. 2013. PMID: 23408105 Free PMC article.
-
6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling.Molecules. 2020 Sep 23;25(19):4374. doi: 10.3390/molecules25194374. Molecules. 2020. PMID: 32977636 Free PMC article.
-
IL6 secreted by Ewing sarcoma tumor microenvironment confers anti-apoptotic and cell-disseminating paracrine responses in Ewing sarcoma cells.BMC Cancer. 2015 Jul 28;15:552. doi: 10.1186/s12885-015-1564-7. BMC Cancer. 2015. PMID: 26215971 Free PMC article.
-
Clinical and Pre-Clinical Evidence of Carbonic Anhydrase IX in Pancreatic Cancer and Its High Expression in Pre-Cancerous Lesions.Cancers (Basel). 2020 Jul 22;12(8):2005. doi: 10.3390/cancers12082005. Cancers (Basel). 2020. PMID: 32707920 Free PMC article. Review.
-
Pro-Inflammatory and Anti-Inflammatory Salivary Cytokines in Breast Cancer: Relationship with Clinicopathological Characteristics of the Tumor.Curr Issues Mol Biol. 2022 Oct 6;44(10):4676-4691. doi: 10.3390/cimb44100319. Curr Issues Mol Biol. 2022. PMID: 36286034 Free PMC article.
References
-
- Hodge D.R., Hurt E.M., Farrar W.L. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer. 2005;41:2502–2512. - PubMed
-
- Rose-John S., Scheller J., Elson G., Jones S.A. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors:role in inflammation and cancer. J. Leukoc. Biol. 2006;80:227–236. - PubMed
-
- Knupfer H., Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res. Treat. 2007;102:129–135. - PubMed
-
- Zhang G.J., Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–1432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

