Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates
- PMID: 18032487
- PMCID: PMC2224460
- DOI: 10.1128/JVI.00450-07
Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates
Abstract
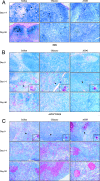
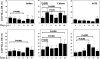
The events that contribute to the progression to AIDS during the acute phase of a primate lentiviral infection are still poorly understood. In this study, we used pathogenic and nonpathogenic simian models of simian immunodeficiency virus (SIV) infection of rhesus macaques (RMs) and African green monkeys (AGMs), respectively, to investigate the relationship between apoptosis in lymph nodes and the extent of viral replication, immune activation, and disease outcome. Here, we show that, in SIVmac251-infected RMs, a marked increased in lymphocyte apoptosis is evident during primary infection at the level of lymph nodes. Interestingly, the levels of apoptosis correlated with the extent of viral replication and the rate of disease progression to AIDS, with higher apoptosis in RMs of Indian genetic background than in those of Chinese origin. In stark contrast, no changes in the levels of lymphocyte apoptosis were observed during primary infection in the nonpathogenic model of SIVagm-sab infection of AGMs, despite similarly high rates of viral replication. A further and early divergence between SIV-infected RMs and AGMs was observed in terms of the dynamics of T- and B-cell proliferation in lymph nodes, with RMs showing significantly higher levels of cycling cells (Ki67(+)) in the T-cell zones in association with relatively low levels of Ki67(+) in the B-cell zones, whereas AGMs displayed a low frequency of Ki67(+) in the T-cell area but a high proportion of Ki67(+) cells in the B-cell area. As such, this study suggests that species-specific host factors determine an early immune response to SIV that predominantly involves either cellular or humoral immunity in RMs and AGMs, respectively. Taken together, these data are consistent with the hypotheses that (i) high levels of T-cell activation and lymphocyte apoptosis are key pathogenic factors during pathogenic SIV infection of RMs and (ii) low T-cell activation and apoptosis are determinants of the AIDS resistance of SIVagm-infected AGMs, despite high levels of SIVagm replication.
Figures


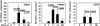


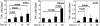
Similar articles
-
Early divergence in neutrophil apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates.J Immunol. 2008 Dec 15;181(12):8613-23. doi: 10.4049/jimmunol.181.12.8613. J Immunol. 2008. PMID: 19050281 Free PMC article.
-
Effect of B-cell depletion on viral replication and clinical outcome of simian immunodeficiency virus infection in a natural host.J Virol. 2009 Oct;83(20):10347-57. doi: 10.1128/JVI.00880-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656874 Free PMC article.
-
Rapid Development of gp120-Focused Neutralizing B Cell Responses during Acute Simian Immunodeficiency Virus Infection of African Green Monkeys.J Virol. 2015 Sep;89(18):9485-98. doi: 10.1128/JVI.01564-15. Epub 2015 Jul 8. J Virol. 2015. PMID: 26157116 Free PMC article.
-
What can natural infection of African monkeys with simian immunodeficiency virus tell us about the pathogenesis of AIDS?AIDS Rev. 2004 Jan-Mar;6(1):40-53. AIDS Rev. 2004. PMID: 15168740 Review.
-
SIVagm: genetic and biological features associated with replication.Front Biosci. 2003 Sep 1;8:d1170-85. doi: 10.2741/1130. Front Biosci. 2003. PMID: 12957815 Review.
Cited by
-
Increased inherent intestinal granzyme B expression may be associated with SIV pathogenesis in Asian non-human primates.J Med Primatol. 2011 Dec;40(6):414-26. doi: 10.1111/j.1600-0684.2011.00482.x. Epub 2011 Jul 7. J Med Primatol. 2011. PMID: 21732950 Free PMC article.
-
HIV/SIV infection primes monocytes and dendritic cells for apoptosis.PLoS Pathog. 2011 Jun;7(6):e1002087. doi: 10.1371/journal.ppat.1002087. Epub 2011 Jun 23. PLoS Pathog. 2011. PMID: 21731488 Free PMC article.
-
CD4(+)CD25(+) T regulatory cells inhibit CD8(+) IFN-gamma production during acute and chronic FIV infection utilizing a membrane TGF-beta-dependent mechanism.AIDS Res Hum Retroviruses. 2010 Feb;26(2):201-16. doi: 10.1089/aid.2009.0162. AIDS Res Hum Retroviruses. 2010. PMID: 20156102 Free PMC article.
-
Early ART reduces viral seeding and innate immunity in liver and lungs of SIV-infected macaques.JCI Insight. 2023 Jul 24;8(14):e167856. doi: 10.1172/jci.insight.167856. JCI Insight. 2023. PMID: 37485876 Free PMC article.
-
HIV-associated chronic immune activation.Immunol Rev. 2013 Jul;254(1):78-101. doi: 10.1111/imr.12079. Immunol Rev. 2013. PMID: 23772616 Free PMC article. Review.
References
-
- Arnoult, D., F. Petit, J. D. Lelievre, D. Lecossier, A. Hance, V. Monceaux, B. Hurtrel, R. Ho Tsong Fang, J. C. Ameisen, and J. Estaquier. 2003. Caspase-dependent and -independent T-cell death pathways in pathogenic simian immunodeficiency virus infection: relationship to disease progression. Cell Death Differ. 101240-1252. - PubMed
-
- Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, S. J. O'Brien, et al. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 2731856-1862. - PubMed
-
- Deeks, S. G., C. M. Kitchen, L. Liu, H. Guo, R. Gascon, A. B. Narvaez, P. Hunt, J. N. Martin, J. O. Kahn, J. Levy, M. S. McGrath, and F. M. Hecht. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104942-947. - PubMed
-
- de Roda Husman, A. M., H. Blaak, M. Brouwer, and H. Schuitemaker. 1999. CC chemokine receptor 5 cell-surface expression in relation to CC chemokine receptor 5 genotype and the clinical course of HIV-1 infection. J. Immunol. 1634597-4603. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

