Breast cancer: origins and evolution
- PMID: 17975657
- PMCID: PMC2045618
- DOI: 10.1172/JCI33295
Breast cancer: origins and evolution
Abstract
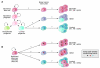
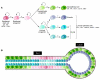
Breast cancer is not a single disease, but rather is composed of distinct subtypes associated with different clinical outcomes. Understanding this heterogeneity is key for the development of targeted cancer-preventative and -therapeutic interventions. Current models explaining inter- and intratumoral diversity are the cancer stem cell and the clonal evolution hypotheses. Although tumor initiation and progression are predominantly driven by acquired genetic alterations, recent data implicate a role for microenvironmental and epigenetic changes as well. Comprehensive unbiased studies of tumors and patient populations have significantly advanced our molecular understanding of breast cancer, but translating these findings into clinical practice remains a challenge.
Figures

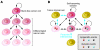
Similar articles
-
Breast cancers, mammary stem cells, and cancer stem cells, characteristics, and hypotheses.Neoplasia. 2020 Dec;22(12):663-678. doi: 10.1016/j.neo.2020.09.009. Epub 2020 Oct 23. Neoplasia. 2020. PMID: 33142233 Free PMC article. Review.
-
Origins of breast cancer subtypes and therapeutic implications.Nat Clin Pract Oncol. 2007 Sep;4(9):516-25. doi: 10.1038/ncponc0908. Nat Clin Pract Oncol. 2007. PMID: 17728710 Review.
-
Role of epithelial stem/progenitor cells in mammary cancer.Gene Expr. 2011;15(3):133-40. doi: 10.3727/105221611x13176664479368. Gene Expr. 2011. PMID: 22268295 Free PMC article. Review.
-
Epigenome remodelling in breast cancer: insights from an early in vitro model of carcinogenesis.Breast Cancer Res. 2012 Nov 15;14(6):215. doi: 10.1186/bcr3237. Breast Cancer Res. 2012. PMID: 23168266 Free PMC article. Review.
-
Application of the D492 Cell Lines to Explore Breast Morphogenesis, EMT and Cancer Progression in 3D Culture.J Mammary Gland Biol Neoplasia. 2019 Jun;24(2):139-147. doi: 10.1007/s10911-018-09424-w. Epub 2019 Jan 25. J Mammary Gland Biol Neoplasia. 2019. PMID: 30684066 Review.
Cited by
-
Deciphering breast cancer dynamics: insights from single-cell and spatial profiling in the multi-omics era.Biomark Res. 2024 Sep 18;12(1):107. doi: 10.1186/s40364-024-00654-1. Biomark Res. 2024. PMID: 39294728 Free PMC article. Review.
-
Novel lncRNAs Co-Expression Networks Identifies LINC00504 with Oncogenic Role in Luminal A Breast Cancer Cells.Int J Mol Sci. 2021 Feb 28;22(5):2420. doi: 10.3390/ijms22052420. Int J Mol Sci. 2021. PMID: 33670895 Free PMC article.
-
Molecular subtypes and imaging phenotypes of breast cancer.Ultrasonography. 2016 Oct;35(4):281-8. doi: 10.14366/usg.16030. Epub 2016 Jul 21. Ultrasonography. 2016. PMID: 27599892 Free PMC article. Review.
-
Increased invasiveness and aggressiveness in breast epithelia with cytoplasmic p63 expression.Int J Biol Sci. 2010 Aug 8;6(5):428-42. doi: 10.7150/ijbs.6.428. Int J Biol Sci. 2010. PMID: 20714441 Free PMC article.
-
The role of miR-31 and its target gene SATB2 in cancer-associated fibroblasts.Cell Cycle. 2010 Nov 1;9(21):4387-98. doi: 10.4161/cc.9.21.13674. Epub 2010 Nov 17. Cell Cycle. 2010. PMID: 20980827 Free PMC article.
References
-
- Kamangar F., Dores G.M., Anderson W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. . 2006;24:2137–2150. - PubMed
-
- Perou C.M., et al. Molecular portraits of human breast tumours. Nature. . 2000;406:747–752. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

