Arterial response to shear stress critically depends on endothelial TRPV4 expression
- PMID: 17786199
- PMCID: PMC1959246
- DOI: 10.1371/journal.pone.0000827
Arterial response to shear stress critically depends on endothelial TRPV4 expression
Abstract
Background: In blood vessels, the endothelium is a crucial signal transduction interface in control of vascular tone and blood pressure to ensure energy and oxygen supply according to the organs' needs. In response to vasoactive factors and to shear stress elicited by blood flow, the endothelium secretes vasodilating or vasocontracting autacoids, which adjust the contractile state of the smooth muscle. In endothelial sensing of shear stress, the osmo- and mechanosensitive Ca(2+)-permeable TRPV4 channel has been proposed to be candidate mechanosensor. Using TRPV4(-/-) mice, we now investigated whether the absence of endothelial TRPV4 alters shear-stress-induced arterial vasodilation.
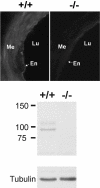
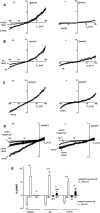
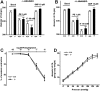
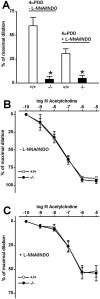
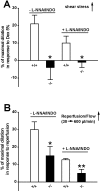
Methodology/principal findings: In TRPV4(-/-) mice, loss of the TRPV4 protein was confirmed by Western blot, immunohistochemistry and by in situ-patch-clamp techniques in carotid artery endothelial cells (CAEC). Endothelium-dependent vasodilation was determined by pressure myography in carotid arteries (CA) from TRPV4(-/-) mice and wild-type littermates (WT). In WT CAEC, TRPV4 currents could be elicited by TRPV4 activators 4alpha-phorbol-12,13-didecanoate (4alphaPDD), arachidonic acid (AA), and by hypotonic cell swelling (HTS). In striking contrast, in TRPV4(-/-) mice, 4alphaPDD did not produce currents and currents elicited by AA and HTS were significantly reduced. 4alphaPDD caused a robust and endothelium-dependent vasodilation in WT mice, again conspicuously absent in TRPV4(-/-) mice. Shear stress-induced vasodilation could readily be evoked in WT, but was completely eliminated in TRPV4(-/-) mice. In addition, flow/reperfusion-induced vasodilation was significantly reduced in TRPV4(-/-) vs. WT mice. Vasodilation in response to acetylcholine, vasoconstriction in response to phenylephrine, and passive mechanical compliance did not differ between genotypes, greatly underscoring the specificity of the above trpv4-dependent phenotype for physiologically relevant shear stress.
Conclusions/significance: Genetically encoded loss-of-function of trpv4 results in a loss of shear stress-induced vasodilation, a response pattern critically dependent on endothelial TRPV4 expression. Thus, Ca(2+)-influx through endothelial TRPV4 channels is a molecular mechanism contributing significantly to endothelial mechanotransduction.
Conflict of interest statement
Figures
Similar articles
-
Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation.Arterioscler Thromb Vasc Biol. 2006 Jul;26(7):1495-502. doi: 10.1161/01.ATV.0000225698.36212.6a. Epub 2006 May 4. Arterioscler Thromb Vasc Biol. 2006. PMID: 16675722
-
Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation.Cardiovasc Res. 2008 Dec 1;80(3):445-52. doi: 10.1093/cvr/cvn207. Epub 2008 Aug 5. Cardiovasc Res. 2008. PMID: 18682435
-
TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress.Am J Physiol Heart Circ Physiol. 2010 Feb;298(2):H466-76. doi: 10.1152/ajpheart.00854.2009. Epub 2009 Dec 4. Am J Physiol Heart Circ Physiol. 2010. PMID: 19966050 Free PMC article.
-
Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels.J Cardiovasc Pharmacol. 2011 Feb;57(2):148-53. doi: 10.1097/FJC.0b013e3181f580d9. J Cardiovasc Pharmacol. 2011. PMID: 20729757 Review.
-
Role of TRPV4 channel in vasodilation and neovascularization.Microcirculation. 2021 Aug;28(6):e12703. doi: 10.1111/micc.12703. Epub 2021 May 24. Microcirculation. 2021. PMID: 33971061 Review.
Cited by
-
Calcium regulation by thermo- and osmosensing transient receptor potential vanilloid channels (TRPVs) in human conjunctival epithelial cells.Histochem Cell Biol. 2012 Jun;137(6):743-61. doi: 10.1007/s00418-012-0924-5. Epub 2012 Feb 12. Histochem Cell Biol. 2012. PMID: 22327830
-
Interdomain interactions control Ca2+-dependent potentiation in the cation channel TRPV4.PLoS One. 2010 May 11;5(5):e10580. doi: 10.1371/journal.pone.0010580. PLoS One. 2010. PMID: 20485495 Free PMC article.
-
Endothelial TRPV4 channels modulate vascular tone by Ca2+ -induced Ca2+ release at inositol 1,4,5-trisphosphate receptors.Br J Pharmacol. 2019 Sep;176(17):3297-3317. doi: 10.1111/bph.14762. Epub 2019 Jul 24. Br J Pharmacol. 2019. PMID: 31177523 Free PMC article.
-
TRP channel Ca(2+) sparklets: fundamental signals underlying endothelium-dependent hyperpolarization.Am J Physiol Cell Physiol. 2013 Nov 15;305(10):C999-C1008. doi: 10.1152/ajpcell.00273.2013. Epub 2013 Sep 11. Am J Physiol Cell Physiol. 2013. PMID: 24025865 Free PMC article. Review.
-
Calcium-activated potassium channels and endothelial dysfunction: therapeutic options?Br J Pharmacol. 2009 Feb;156(4):545-62. doi: 10.1111/j.1476-5381.2009.00052.x. Epub 2009 Jan 29. Br J Pharmacol. 2009. PMID: 19187341 Free PMC article. Review.
References
-
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. - PubMed
-
- Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. - PubMed
-
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. - PubMed
-
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. - PubMed
-
- Köhler R, Hoyer J. The endothelium-derived hyperpolarizing factor: insights from genetic animal models. Kidney Int Apr. 2007;25; [Epub ahead of print]; doi:10.1038/sj.ki.5002303 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

