Vector integration is nonrandom and clustered and influences the fate of lymphopoiesis in SCID-X1 gene therapy
- PMID: 17671652
- PMCID: PMC1934585
- DOI: 10.1172/JCI31659
Vector integration is nonrandom and clustered and influences the fate of lymphopoiesis in SCID-X1 gene therapy
Abstract
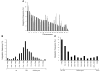
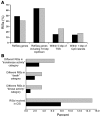
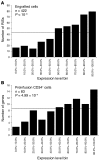
Recent reports have challenged the notion that retroviruses and retroviral vectors integrate randomly into the host genome. These reports pointed to a strong bias toward integration in and near gene coding regions and, for gammaretroviral vectors, around transcription start sites. Here, we report the results obtained from a large-scale mapping of 572 retroviral integration sites (RISs) isolated from cells of 9 patients with X-linked SCID (SCID-X1) treated with a retrovirus-based gene therapy protocol. Our data showed that two-thirds of insertions occurred in or very near to genes, of which more than half were highly expressed in CD34(+) progenitor cells. Strikingly, one-fourth of all integrations were clustered as common integration sites (CISs). The highly significant incidence of CISs in circulating T cells and the nature of their locations indicate that insertion in many gene loci has an influence on cell engraftment, survival, and proliferation. Beyond the observed cases of insertional mutagenesis in 3 patients, these data help to elucidate the relationship between vector insertion and long-term in vivo selection of transduced cells in human patients with SCID-X1.
Figures
Comment in
-
Retroviral integration and human gene therapy.J Clin Invest. 2007 Aug;117(8):2083-6. doi: 10.1172/JCI32949. J Clin Invest. 2007. PMID: 17671645 Free PMC article.
Similar articles
-
Retroviral integration and human gene therapy.J Clin Invest. 2007 Aug;117(8):2083-6. doi: 10.1172/JCI32949. J Clin Invest. 2007. PMID: 17671645 Free PMC article.
-
Gammaretrovirus-mediated correction of SCID-X1 is associated with skewed vector integration site distribution in vivo.J Clin Invest. 2007 Aug;117(8):2241-9. doi: 10.1172/JCI31661. J Clin Invest. 2007. PMID: 17671654 Free PMC article. Clinical Trial.
-
Dynamics of gene-modified progenitor cells analyzed by tracking retroviral integration sites in a human SCID-X1 gene therapy trial.Blood. 2010 Jun 3;115(22):4356-66. doi: 10.1182/blood-2009-12-257352. Epub 2010 Mar 12. Blood. 2010. PMID: 20228274 Free PMC article. Clinical Trial.
-
Gene Therapy for X-Linked Severe Combined Immunodeficiency: Where Do We Stand?Hum Gene Ther. 2016 Feb;27(2):108-16. doi: 10.1089/hum.2015.137. Hum Gene Ther. 2016. PMID: 26790362 Free PMC article. Review.
-
Stem cell gene transfer: insights into integration and hematopoiesis from primate genetic marking studies.Ann N Y Acad Sci. 2005 Jun;1044:178-82. doi: 10.1196/annals.1349.023. Ann N Y Acad Sci. 2005. PMID: 15958711 Review.
Cited by
-
Long-term follow-up of foamy viral vector-mediated gene therapy for canine leukocyte adhesion deficiency.Mol Ther. 2013 May;21(5):964-72. doi: 10.1038/mt.2013.34. Epub 2013 Mar 26. Mol Ther. 2013. PMID: 23531552 Free PMC article.
-
Development of gene therapy for thalassemia.Cold Spring Harb Perspect Med. 2012 Nov 1;2(11):a011833. doi: 10.1101/cshperspect.a011833. Cold Spring Harb Perspect Med. 2012. PMID: 23125203 Free PMC article. Review.
-
Counting stem cells: methodological constraints.Nat Methods. 2012 May 30;9(6):567-74. doi: 10.1038/nmeth.2043. Nat Methods. 2012. PMID: 22669654
-
TALEN/CRISPR-mediated engineering of a promoterless anti-viral RNAi hairpin into an endogenous miRNA locus.Nucleic Acids Res. 2017 Jan 9;45(1):e3. doi: 10.1093/nar/gkw805. Epub 2016 Sep 9. Nucleic Acids Res. 2017. PMID: 27614072 Free PMC article.
-
Assessing the risk of T-cell malignancies in mouse models of SCID-X1.Mol Ther. 2010 May;18(5):868-70. doi: 10.1038/mt.2010.69. Mol Ther. 2010. PMID: 20436493 Free PMC article. No abstract available.
References
-
- Cavazzana-Calvo M., et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. - PubMed
-
- Aiuti A., et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. - PubMed
-
- Gaspar H.B., et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–2187. - PubMed
-
- Coffin, J.M., Hughes, S.H., and Varmus, H.E. 1997.Retroviruses . Cold Spring Harbor Laboratory Press. Plainview, New York, USA. 843 pp. - PubMed
-
- Moolten F.L., Cupples L.A. A model for predicting the risk of cancer consequent to retroviral gene therapy. Hum. Gene Ther. 1992;3:479–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

