Reducing agents sensitize C-type nociceptors by relieving high-affinity zinc inhibition of T-type calcium channels
- PMID: 17670971
- PMCID: PMC6673068
- DOI: 10.1523/JNEUROSCI.1800-07.2007
Reducing agents sensitize C-type nociceptors by relieving high-affinity zinc inhibition of T-type calcium channels
Abstract
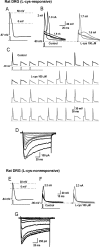
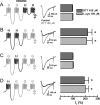
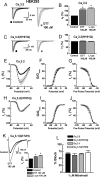
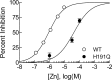
Recent studies have demonstrated an important role for T-type Ca2+ channels (T-channels) in controlling the excitability of peripheral pain-sensing neurons (nociceptors). However, the molecular mechanisms underlying the functions of T-channels in nociceptors are poorly understood. Here, we demonstrate that reducing agents as well as endogenous metal chelators sensitize C-type dorsal root ganglion nociceptors by chelating Zn2+ ions off specific extracellular histidine residues on Ca(v)3.2 T-channels, thus relieving tonic channel inhibition, enhancing Ca(v)3.2 currents, and lowering the threshold for nociceptor excitability in vitro and in vivo. Collectively, these findings describe a novel mechanism of nociceptor sensitization and firmly establish reducing agents, as well as Zn2+, Zn2+-chelating amino acids, and Zn2+-chelating proteins as endogenous modulators of Ca(v)3.2 and nociceptor excitability.
Figures



Similar articles
-
The endogenous redox agent L-cysteine induces T-type Ca2+ channel-dependent sensitization of a novel subpopulation of rat peripheral nociceptors.J Neurosci. 2005 Sep 21;25(38):8766-75. doi: 10.1523/JNEUROSCI.2527-05.2005. J Neurosci. 2005. PMID: 16177046 Free PMC article.
-
Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability.J Physiol. 2016 Jul 1;594(13):3561-74. doi: 10.1113/JP271925. Epub 2016 May 7. J Physiol. 2016. PMID: 26931411 Free PMC article.
-
Redox modulation of T-type calcium channels in rat peripheral nociceptors.Neuron. 2001 Jul 19;31(1):75-85. doi: 10.1016/s0896-6273(01)00338-5. Neuron. 2001. PMID: 11498052
-
Targeting Ca2+ channels to treat pain: T-type versus N-type.Trends Pharmacol Sci. 2004 Sep;25(9):465-70. doi: 10.1016/j.tips.2004.07.004. Trends Pharmacol Sci. 2004. PMID: 15559248 Review.
-
Is there a role for T-type calcium channels in peripheral and central pain sensitization?Mol Neurobiol. 2006 Dec;34(3):243-8. doi: 10.1385/MN:34:3:243. Mol Neurobiol. 2006. PMID: 17308355 Review.
Cited by
-
TRPA1 has a key role in the somatic pro-nociceptive actions of hydrogen sulfide.PLoS One. 2012;7(10):e46917. doi: 10.1371/journal.pone.0046917. Epub 2012 Oct 11. PLoS One. 2012. PMID: 23071662 Free PMC article.
-
Reversal of neuropathic pain in diabetes by targeting glycosylation of Ca(V)3.2 T-type calcium channels.Diabetes. 2013 Nov;62(11):3828-38. doi: 10.2337/db13-0813. Epub 2013 Jul 8. Diabetes. 2013. PMID: 23835327 Free PMC article.
-
Zinc induces long-term upregulation of T-type calcium current in hippocampal neurons in vivo.J Physiol. 2012 Nov 15;590(22):5895-905. doi: 10.1113/jphysiol.2012.242537. Epub 2012 Aug 28. J Physiol. 2012. PMID: 22930274 Free PMC article.
-
Hydrogen sulfide-induced itch requires activation of Cav3.2 T-type calcium channel in mice.Sci Rep. 2015 Nov 25;5:16768. doi: 10.1038/srep16768. Sci Rep. 2015. PMID: 26602811 Free PMC article.
-
Redox and trace metal regulation of ion channels in the pain pathway.Biochem J. 2015 Sep 15;470(3):275-80. doi: 10.1042/BJ20150522. Biochem J. 2015. PMID: 26341484 Free PMC article. Review.
References
-
- Bhave G, Gereau RW., IV Posttranslational mechanisms of peripheral sensitization. J Neurobiol. 2004;61:88–106. - PubMed
-
- Cardenas CG, Del Mar LP, Scroggs RS. Variation in serotonergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J Neurophysiol. 1995;74:1870–1879. - PubMed
-
- Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302:1416–1418. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

