Mitochondrial uncouplers with an extraordinary dynamic range
- PMID: 17608618
- PMCID: PMC2267406
- DOI: 10.1042/BJ20070606
Mitochondrial uncouplers with an extraordinary dynamic range
Abstract
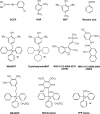
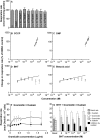
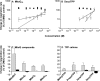
We have discovered that some weak uncouplers (typified by butylated hydroxytoluene) have a dynamic range of more than 10(6) in vitro: the concentration giving measurable uncoupling is less than one millionth of the concentration causing full uncoupling. They achieve this through a high-affinity interaction with the mitochondrial adenine nucleotide translocase that causes significant but limited uncoupling at extremely low uncoupler concentrations, together with more conventional uncoupling at much higher concentrations. Uncoupling at the translocase is not by a conventional weak acid/anion cycling mechanism since it is also caused by substituted triphenylphosphonium molecules, which are not anionic and cannot protonate. Covalent attachment of the uncoupler to a mitochondrially targeted hydrophobic cation sensitizes it to membrane potential, giving a small additional effect. The wide dynamic range of these uncouplers in isolated mitochondria and intact cells reveals a novel allosteric activation of proton transport through the adenine nucleotide translocase and provides a promising starting point for designing safer uncouplers for obesity therapy.
Figures


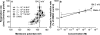



Similar articles
-
Mitochondrial uncoupling caused by a wide variety of protonophores is differently sensitive to carboxyatractyloside in rat heart and liver mitochondria.Biochim Biophys Acta Bioenerg. 2024 Nov 1;1865(4):149506. doi: 10.1016/j.bbabio.2024.149506. Epub 2024 Aug 20. Biochim Biophys Acta Bioenerg. 2024. PMID: 39168228
-
Alkyl esters of 7-hydroxycoumarin-3-carboxylic acid as potent tissue-specific uncouplers of oxidative phosphorylation: Involvement of ATP/ADP translocase in mitochondrial uncoupling.Arch Biochem Biophys. 2022 Oct 15;728:109366. doi: 10.1016/j.abb.2022.109366. Epub 2022 Jul 22. Arch Biochem Biophys. 2022. PMID: 35878680
-
The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria.Eur J Biochem. 1989 Jul 1;182(3):585-92. doi: 10.1111/j.1432-1033.1989.tb14867.x. Eur J Biochem. 1989. PMID: 2546761
-
Uncouplers of oxidative phosphorylation.Environ Health Perspect. 1990 Jul;87:213-8. doi: 10.1289/ehp.9087213. Environ Health Perspect. 1990. PMID: 2176586 Free PMC article. Review.
-
Mitochondrial ATP-Pi exchange complex and the site of uncoupling of oxidative phosphorylation.Fed Proc. 1975 Jul;34(8):1699-706. Fed Proc. 1975. PMID: 1093889 Review.
Cited by
-
Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane.Mol Metab. 2013 Nov 28;3(2):114-23. doi: 10.1016/j.molmet.2013.11.005. eCollection 2014 Apr. Mol Metab. 2013. PMID: 24634817 Free PMC article.
-
The on-off switches of the mitochondrial uncoupling proteins.Trends Biochem Sci. 2010 May;35(5):298-307. doi: 10.1016/j.tibs.2009.11.001. Epub 2009 Dec 16. Trends Biochem Sci. 2010. PMID: 20006514 Free PMC article. Review.
-
Expression of the Cyanobacterial FoF1 ATP Synthase Regulator AtpΘ Depends on Small DNA-Binding Proteins and Differential mRNA Stability.Microbiol Spectr. 2022 Jun 29;10(3):e0256221. doi: 10.1128/spectrum.02562-21. Epub 2022 Apr 21. Microbiol Spectr. 2022. PMID: 35446123 Free PMC article.
-
FBS/BSA media concentration determines CCCP's ability to depolarize mitochondria and activate PINK1-PRKN mitophagy.Autophagy. 2019 Nov;15(11):2002-2011. doi: 10.1080/15548627.2019.1603549. Epub 2019 May 7. Autophagy. 2019. PMID: 31060423 Free PMC article.
-
Use the Protonmotive Force: Mitochondrial Uncoupling and Reactive Oxygen Species.J Mol Biol. 2018 Oct 19;430(21):3873-3891. doi: 10.1016/j.jmb.2018.03.025. Epub 2018 Apr 4. J Mol Biol. 2018. PMID: 29626541 Free PMC article. Review.
References
-
- Harper J. A., Dickinson K., Brand M. D. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes. Rev. 2001;2:255–265. - PubMed
-
- Van Gaal L. F., Rissanen A. M., Scheen A. J., Ziegler O., Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. - PubMed
-
- Halford J. C. Obesity drugs in clinical development. Curr. Opin. Investig. Drugs. 2006;7:312–318. - PubMed
-
- Cutting W. C., Mehrtens H. G., Tainter M. L. Actions and uses of dinitrophenol. JAMA, J. Am. Med. Assoc. 1933;101:193–195.
-
- Tainter M. L., Stockton A. B., Cutting W. C. Dinitrophenol in the treatment of obesity: final report. JAMA, J. Am. Med. Assoc. 1935;105:332–336.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

