Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer
- PMID: 17603493
- PMCID: PMC2135607
- DOI: 10.1038/nm1609
Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer
Abstract
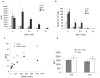
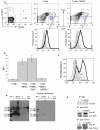
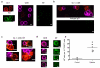
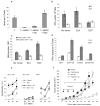
Antigen-specific CD8+ T-cell tolerance, induced by myeloid-derived suppressor cells (MDSCs), is one of the main mechanisms of tumor escape. Using in vivo models, we show here that MDSCs directly disrupt the binding of specific peptide-major histocompatibility complex (pMHC) dimers to CD8-expressing T cells through nitration of tyrosines in a T-cell receptor (TCR)-CD8 complex. This process makes CD8-expressing T cells unable to bind pMHC and to respond to the specific peptide, although they retain their ability to respond to nonspecific stimulation. Nitration of TCR-CD8 is induced by MDSCs through hyperproduction of reactive oxygen species and peroxynitrite during direct cell-cell contact. Molecular modeling suggests specific sites of nitration that might affect the conformational flexibility of TCR-CD8 and its interaction with pMHC. These data identify a previously unknown mechanism of T-cell tolerance in cancer that is also pertinent to many pathological conditions associated with accumulation of MDSCs.
Figures


Similar articles
-
Mechanism of T cell tolerance induced by myeloid-derived suppressor cells.J Immunol. 2010 Mar 15;184(6):3106-16. doi: 10.4049/jimmunol.0902661. Epub 2010 Feb 8. J Immunol. 2010. PMID: 20142361 Free PMC article.
-
Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway.Gastroenterology. 2008 Sep;135(3):871-81, 881.e1-5. doi: 10.1053/j.gastro.2008.06.032. Epub 2008 Jun 12. Gastroenterology. 2008. PMID: 18674538
-
Spatial coordination of CD8 and TCR molecules controls antigen recognition by CD8+ T-cells.FEBS Lett. 2005 Jun 13;579(15):3336-41. doi: 10.1016/j.febslet.2005.04.025. Epub 2005 Apr 26. FEBS Lett. 2005. PMID: 15913613 Review.
-
The duration of TCR/pMHC interactions regulates CTL effector function and tumor-killing capacity.Eur J Immunol. 2009 Aug;39(8):2259-69. doi: 10.1002/eji.200939341. Eur J Immunol. 2009. PMID: 19637198
-
Tumor escape mechanism governed by myeloid-derived suppressor cells.Cancer Res. 2008 Apr 15;68(8):2561-3. doi: 10.1158/0008-5472.CAN-07-6229. Cancer Res. 2008. PMID: 18413722 Review.
Cited by
-
Peroxynitrite promotes immune evasion by reducing tumor antigenicity.Cell Rep Med. 2022 Oct 18;3(10):100787. doi: 10.1016/j.xcrm.2022.100787. Cell Rep Med. 2022. PMID: 36260983 Free PMC article.
-
The Functional Roles of MDSCs in Severe COVID-19 Pathogenesis.Viruses. 2023 Dec 23;16(1):27. doi: 10.3390/v16010027. Viruses. 2023. PMID: 38257728 Free PMC article. Review.
-
Yu-Ping-Feng Formula Exerts Antilung Cancer Effects by Remodeling the Tumor Microenvironment through Regulating Myeloid-Derived Suppressor Cells.Evid Based Complement Alternat Med. 2021 Apr 20;2021:6624461. doi: 10.1155/2021/6624461. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33986819 Free PMC article.
-
Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy.Oncoimmunology. 2015 Feb 3;4(1):e954829. doi: 10.4161/21624011.2014.954829. eCollection 2015 Jan. Oncoimmunology. 2015. PMID: 25949858 Free PMC article. Review.
-
CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function.Cell Rep. 2015 Jul 14;12(2):244-57. doi: 10.1016/j.celrep.2015.06.024. Epub 2015 Jul 2. Cell Rep. 2015. PMID: 26146082 Free PMC article.
References
-
- Pardoll D. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 2003;21:807–839. - PubMed
-
- Sotomayor EM, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat. Med. 1999;5:780–787. - PubMed
-
- Cuenca A, et al. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer. Res. 2003;63:9007–9015. - PubMed
-
- Huang B, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer. Res. 2006;66:1123–1131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

