Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2
- PMID: 17522215
- PMCID: PMC1951286
- DOI: 10.1128/JVI.00403-07
Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2
Abstract
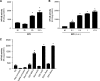
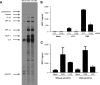
Epstein-Barr virus (EBV) is a gammaherpesvirus infecting the majority of the human adult population in the world. TLR2, a member of the Toll-like receptor (TLR) family, has been implicated in the immune responses to different viruses including members of the herpesvirus family, such as human cytomegalovirus, herpes simplex virus type 1, and varicella-zoster virus. In this report, we demonstrate that infectious and UV-inactivated EBV virions lead to the activation of NF-kappaB through TLR2 using HEK293 cells cotransfected with TLR2-expressing vector along with NF-kappaB-Luc reporter plasmid. NF-kappaB activation in HEK293-TLR2 cells (HEK293 cells transfected with TLR2) by EBV was not enhanced by the presence of CD14. The effect of EBV was abrogated by pretreating HEK293-TLR2 cells with blocking anti-TLR2 antibodies or by preincubating viral particles with neutralizing anti-EBV antibodies 72A1. In addition, EBV infection of primary human monocytes induced the release of MCP-1 (monocyte chemotactic protein 1), and the use of small interfering RNA targeting TLR2 significantly reduced such a chemokine response to EBV. Taken together, these results indicate that TLR2 may be an important pattern recognition receptor in the immune response directed against EBV infection.
Figures
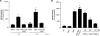
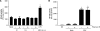
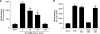
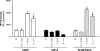
Similar articles
-
Lactoferrin suppresses the Epstein-Barr virus-induced inflammatory response by interfering with pattern recognition of TLR2 and TLR9.Lab Invest. 2014 Nov;94(11):1188-99. doi: 10.1038/labinvest.2014.105. Epub 2014 Jul 28. Lab Invest. 2014. PMID: 25068657
-
TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells.J Immunol. 2010 Sep 15;185(6):3620-31. doi: 10.4049/jimmunol.0903736. Epub 2010 Aug 16. J Immunol. 2010. PMID: 20713890
-
Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2.J Virol. 2005 Oct;79(20):12658-66. doi: 10.1128/JVI.79.20.12658-12666.2005. J Virol. 2005. PMID: 16188968 Free PMC article.
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
-
Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses.Dermatology. 2005;211(3):193-8. doi: 10.1159/000087011. Dermatology. 2005. PMID: 16205063 Review.
Cited by
-
Assessment of the effect of TLR7/8, TLR9 agonists and CD40 ligand on the transformation efficiency of Epstein-Barr virus in human B lymphocytes by limiting dilution assay.Cytotechnology. 2014 Jan;66(1):95-105. doi: 10.1007/s10616-013-9542-x. Epub 2013 Feb 13. Cytotechnology. 2014. PMID: 23404520 Free PMC article.
-
Review on Toll-Like Receptor Activation in Myasthenia Gravis: Application to the Development of New Experimental Models.Clin Rev Allergy Immunol. 2017 Feb;52(1):133-147. doi: 10.1007/s12016-016-8549-4. Clin Rev Allergy Immunol. 2017. PMID: 27207173 Review.
-
How Does Epstein-Barr Virus Contribute to Chronic Periodontitis?Int J Mol Sci. 2020 Mar 12;21(6):1940. doi: 10.3390/ijms21061940. Int J Mol Sci. 2020. PMID: 32178406 Free PMC article. Review.
-
Recognition of herpesviruses by the innate immune system.Nat Rev Immunol. 2011 Feb;11(2):143-54. doi: 10.1038/nri2937. Nat Rev Immunol. 2011. PMID: 21267015 Free PMC article. Review.
-
Toll-like receptors and their role in carcinogenesis and anti-tumor treatment.Cell Mol Biol Lett. 2009;14(2):248-72. doi: 10.2478/s11658-008-0048-z. Epub 2008 Dec 18. Cell Mol Biol Lett. 2009. PMID: 19096763 Free PMC article. Review.
References
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. - PubMed
-
- Aravalli, R. N., S. Hu, T. N. Rowen, J. M. Palmquist, and J. R. Lokensgard. 2005. TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 175:4189-4193. - PubMed
-
- Beaulieu, A. D., R. Paquin, and J. Gosselin. 1995. Epstein-Barr virus modulates de novo protein synthesis in human neutrophils. Blood 86:2789-2798. - PubMed
-
- Beutler, B., Z. Jiang, P. Georgel, K. Crozat, B. Croker, S. Rutschmann, X. Du, and K. Hoebe. 2006. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 24:353-389. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

