Truncated form of the Epstein-Barr virus protein EBNA-LP protects against caspase-dependent apoptosis by inhibiting protein phosphatase 2A
- PMID: 17494066
- PMCID: PMC1933342
- DOI: 10.1128/JVI.02435-06
Truncated form of the Epstein-Barr virus protein EBNA-LP protects against caspase-dependent apoptosis by inhibiting protein phosphatase 2A
Abstract
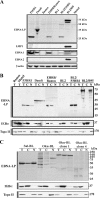
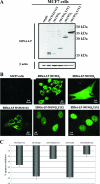
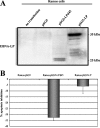
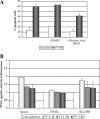
The Epstein-Barr virus (EBV)-encoded leader protein, EBNA-LP, strongly activates the EBNA2-mediated transcriptional activation of cellular and viral genes and is therefore important for EBV-induced B-cell transformation. However, a truncated form of EBNA-LP is produced in cells infected with variant EBV strains lacking EBNA2 due to a genetic deletion. The function of this truncated form is unknown. We show here that some Burkitt's lymphoma cells harboring defective EBV strains are specifically resistant to the caspase-dependent apoptosis induced by verotoxin 1 (VT-1) or staurosporine. These cells produced low-molecular-weight Y1Y2-truncated isoforms of EBNA-LP, which were partly localized in the cytoplasm. The transfection of sensitive cells with constructs encoding truncated EBNA-LP isoforms, but not full-length EBNA-LP, induced resistance to caspase-mediated apoptosis. Furthermore, VT-1 induced protein phosphatase 2A (PP2A) activation in sensitive cells but not in resistant cells, in which the truncated EBNA-LP interacted with this protein. Thus, the resistance to apoptosis observed in cells harboring defective EBV strains most probably results from the inactivation of PP2A via interactions with low-molecular-weight Y1Y2-truncated EBNA-LP isoforms.
Figures
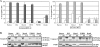
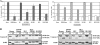
Similar articles
-
New Interactors of the Truncated EBNA-LP Protein Identified by Mass Spectrometry in P3HR1 Burkitt's Lymphoma Cells.Cancers (Basel). 2018 Jan 5;10(1):12. doi: 10.3390/cancers10010012. Cancers (Basel). 2018. PMID: 29303964 Free PMC article.
-
The Epstein-Barr virus EBNA-LP protein preferentially coactivates EBNA2-mediated stimulation of latent membrane proteins expressed from the viral divergent promoter.J Virol. 2005 Apr;79(7):4492-505. doi: 10.1128/JVI.79.7.4492-4505.2005. J Virol. 2005. PMID: 15767449 Free PMC article.
-
Genetic analysis of the Epstein-Barr virus-coded leader protein EBNA-LP as a co-activator of EBNA2 function.J Gen Virol. 2001 Dec;82(Pt 12):3067-3079. doi: 10.1099/0022-1317-82-12-3067. J Gen Virol. 2001. PMID: 11714985
-
EBNA2 and Its Coactivator EBNA-LP.Curr Top Microbiol Immunol. 2015;391:35-59. doi: 10.1007/978-3-319-22834-1_2. Curr Top Microbiol Immunol. 2015. PMID: 26428371 Review.
-
T cell recognition of Epstein-Barr virus associated lymphomas.Cancer Surv. 1992;13:53-80. Cancer Surv. 1992. PMID: 1330300 Review.
Cited by
-
New Interactors of the Truncated EBNA-LP Protein Identified by Mass Spectrometry in P3HR1 Burkitt's Lymphoma Cells.Cancers (Basel). 2018 Jan 5;10(1):12. doi: 10.3390/cancers10010012. Cancers (Basel). 2018. PMID: 29303964 Free PMC article.
-
Nuclear-cytoplasmic shuttling is not required for the Epstein-Barr virus EBNA-LP transcriptional coactivation function.J Virol. 2009 Jul;83(14):7109-16. doi: 10.1128/JVI.00654-09. Epub 2009 Apr 29. J Virol. 2009. PMID: 19403674 Free PMC article.
-
Proteomic approaches to investigate gammaherpesvirus biology and associated tumorigenesis.Adv Virus Res. 2021;109:201-254. doi: 10.1016/bs.aivir.2020.10.001. Epub 2020 Nov 9. Adv Virus Res. 2021. PMID: 33934828 Free PMC article. Review.
-
Schizophrenia is Associated With an Aberrant Immune Response to Epstein-Barr Virus.Schizophr Bull. 2019 Sep 11;45(5):1112-1119. doi: 10.1093/schbul/sby164. Schizophr Bull. 2019. PMID: 30462333 Free PMC article.
-
Proof for EBV's sustaining role in Burkitt's lymphomas.Semin Cancer Biol. 2009 Dec;19(6):389-93. doi: 10.1016/j.semcancer.2009.07.006. Epub 2009 Jul 21. Semin Cancer Biol. 2009. PMID: 19628040 Free PMC article. Review.
References
-
- Alfieri, C., M. Birkenbach, and E. Kieff. 1991. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology 181:595-608. - PubMed
-
- Alvarado-Kristensson, M., and T. Andersson. 2005. Protein phosphatase 2A regulates apoptosis in neutrophils by dephosphorylating both p38 MAPK and its substrate caspase 3. J. Biol. Chem. 280:6238-6244. - PubMed
-
- Chen, W., and W. C. Hahn. 2003. SV40 early region oncoproteins and human cell transformation. Histol. Histopathol. 18:541-550. - PubMed
-
- Chen, W., R. Possemato, K. T. Campbell, C. A. Plattner, D. C. Pallas, and W. C. Hahn. 2004. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5:127-136. - PubMed
-
- Defrance, T. 2005. Mature B cells: apoptosis checkpoints. Transplantation 79:S4-S7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

