Detection of the abundance of diacylglycerol and triacylglycerol molecular species in cells using neutral loss mass spectrometry
- PMID: 17442253
- PMCID: PMC2034497
- DOI: 10.1016/j.ab.2007.03.012
Detection of the abundance of diacylglycerol and triacylglycerol molecular species in cells using neutral loss mass spectrometry
Abstract
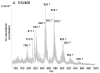
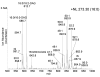
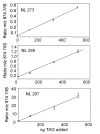
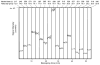
Triacylglycerols (TAGs) are neutral lipids present in all mammalian cells as energy reserves, and diacylglycerols (DAGs) are present as intermediates in phospholipid biosynthesis and as signaling molecules. The molecular species of TAGs and DAGs present in mammalian cells are quite complex, and previous investigations revealed multiple isobaric species having molecular weights at virtually every even mass between 600 and 900 Da, making it difficult to assess changes of individual molecular species after cell activation. A method has been developed, using tandem MS and neutral loss scanning, to quantitatively analyze changes in those glyceryl ester molecular species containing identical fatty acyl groups. This was carried out by neutral loss scanning of 18 common fatty acyl groups where the neutral loss corresponded to the free carboxylic acid plus NH(3). Deuterium-labeled internal standards were used to normalize the signal for each nominal [M+NH(4)](+) ion undergoing this neutral loss reaction. This method was applied in studies of TAGs in RAW 264.7 cells treated with the toll-like receptor 4 ligand Kdo(2)-lipid A. A 50:1-TAG containing 18:1 was found to increase significantly over a 24-h time course after Kdo(2)-lipid A exposure, whereas an isobaric 50:1-TAG containing 16:1 did not change relative to controls.
Figures







Similar articles
-
Qualitative analysis and quantitative assessment of changes in neutral glycerol lipid molecular species within cells.Methods Enzymol. 2007;432:1-20. doi: 10.1016/S0076-6879(07)32001-6. Methods Enzymol. 2007. PMID: 17954211
-
Evaluation of triacylglycerol (TAG) profiles and their contents in salmon muscle tissue using ESI-MS/MS spectrometry with multiple neutral loss scans.Food Chem. 2020 Sep 15;324:126816. doi: 10.1016/j.foodchem.2020.126816. Epub 2020 Apr 15. Food Chem. 2020. PMID: 32344337
-
Direct qualitative analysis of triacylglycerols by electrospray mass spectrometry using a linear ion trap.J Am Soc Mass Spectrom. 2005 Sep;16(9):1498-1509. doi: 10.1016/j.jasms.2005.04.017. J Am Soc Mass Spectrom. 2005. PMID: 16019221
-
Chemistry and liquid chromatography methods for the analyses of primary oxidation products of triacylglycerols.Free Radic Res. 2015 May;49(5):549-64. doi: 10.3109/10715762.2015.1022540. Epub 2015 Mar 31. Free Radic Res. 2015. PMID: 25824968 Review.
-
Amended final report on the safety assessment of glyceryl dilaurate, glyceryl diarachidate, glyceryl dibehenate, glyceryl dierucate, glyceryl dihydroxystearate, glyceryl diisopalmitate, glyceryl diisostearate, glyceryl dilinoleate, glyceryl dimyristate, glyceryl dioleate, glyceryl diricinoleate, glyceryl dipalmitate, glyceryl dipalmitoleate, glyceryl distearate, glyceryl palmitate lactate, glyceryl stearate citrate, glyceryl stearate lactate, and glyceryl stearate succinate.Int J Toxicol. 2007;26 Suppl 3:1-30. doi: 10.1080/10915810701663143. Int J Toxicol. 2007. PMID: 18273450 Review.
Cited by
-
The Diapause Lipidomes of Three Closely Related Beetle Species Reveal Mechanisms for Tolerating Energetic and Cold Stress in High-Latitude Seasonal Environments.Front Physiol. 2020 Sep 25;11:576617. doi: 10.3389/fphys.2020.576617. eCollection 2020. Front Physiol. 2020. PMID: 33101058 Free PMC article.
-
In-depth triacylglycerol profiling using MS3 Q-Trap mass spectrometry.Anal Chim Acta. 2021 Nov 1;1184:339023. doi: 10.1016/j.aca.2021.339023. Epub 2021 Sep 3. Anal Chim Acta. 2021. PMID: 34625255 Free PMC article.
-
Glycerolipid and cholesterol ester analyses in biological samples by mass spectrometry.Biochim Biophys Acta. 2011 Nov;1811(11):776-83. doi: 10.1016/j.bbalip.2011.06.019. Epub 2011 Jun 26. Biochim Biophys Acta. 2011. PMID: 21757029 Free PMC article. Review.
-
Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes.PLoS One. 2013 Sep 27;8(9):e74341. doi: 10.1371/journal.pone.0074341. eCollection 2013. PLoS One. 2013. PMID: 24086336 Free PMC article.
-
Genetic correlation of the plasma lipidome with type 2 diabetes, prediabetes and insulin resistance in Mexican American families.BMC Genet. 2017 May 19;18(1):48. doi: 10.1186/s12863-017-0515-5. BMC Genet. 2017. PMID: 28525987 Free PMC article.
References
-
- Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134–176. - PubMed
-
- Brown DA. Lipid droplets: proteins floating on a pool of fat. Curr Biol. 2001;5:R446–R449. - PubMed
-
- Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–3792. - PubMed
-
- Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R. Lipolysis: pathway under construction. Curr Opin Lipidol. 2005;16:333–340. - PubMed
-
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

