Systematic identification of cellular signals reactivating Kaposi sarcoma-associated herpesvirus
- PMID: 17397260
- PMCID: PMC1839163
- DOI: 10.1371/journal.ppat.0030044
Systematic identification of cellular signals reactivating Kaposi sarcoma-associated herpesvirus
Abstract
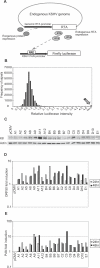
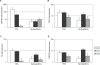
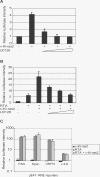
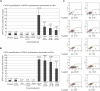
The herpesvirus life cycle has two distinct phases: latency and lytic replication. The balance between these two phases is critical for viral pathogenesis. It is believed that cellular signals regulate the switch from latency to lytic replication. To systematically evaluate the cellular signals regulating this reactivation process in Kaposi sarcoma-associated herpesvirus, the effects of 26,000 full-length cDNA expression constructs on viral reactivation were individually assessed in primary effusion lymphoma-derived cells that harbor the latent virus. A group of diverse cellular signaling proteins were identified and validated in their effect of inducing viral lytic gene expression from the latent viral genome. The results suggest that multiple cellular signaling pathways can reactivate the virus in a genetically homogeneous cell population. Further analysis revealed that the Raf/MEK/ERK/Ets-1 pathway mediates Ras-induced reactivation. The same pathway also mediates spontaneous reactivation, which sets the first example to our knowledge of a specific cellular pathway being studied in the spontaneous reactivation process. Our study provides a functional genomic approach to systematically identify the cellular signals regulating the herpesvirus life cycle, thus facilitating better understanding of a fundamental issue in virology and identifying novel therapeutic targets.
Conflict of interest statement
Figures


Similar articles
-
Intracellular-activated Notch1 can reactivate Kaposi's sarcoma-associated herpesvirus from latency.Virology. 2006 Aug 1;351(2):393-403. doi: 10.1016/j.virol.2006.03.047. Epub 2006 May 15. Virology. 2006. PMID: 16701788
-
Anti-HIV agent MAP30 modulates the expression profile of viral and cellular genes for proliferation and apoptosis in AIDS-related lymphoma cells infected with Kaposi's sarcoma-associated virus.Biochem Biophys Res Commun. 2001 Oct 5;287(4):983-94. doi: 10.1006/bbrc.2001.5689. Biochem Biophys Res Commun. 2001. PMID: 11573962
-
Signaling cascades triggered by bacterial metabolic end products during reactivation of Kaposi's sarcoma-associated herpesvirus.J Virol. 2007 Jun;81(11):6032-42. doi: 10.1128/JVI.02504-06. Epub 2007 Mar 21. J Virol. 2007. PMID: 17376930 Free PMC article.
-
Targeting the PI3K and MAPK pathways to treat Kaposi's-sarcoma-associated herpes virus infection and pathogenesis.Expert Opin Ther Targets. 2007 May;11(5):589-99. doi: 10.1517/14728222.11.5.589. Expert Opin Ther Targets. 2007. PMID: 17465719 Review.
-
Kaposi's sarcoma-associated herpesvirus (KSHV/HHV8): key aspects of epidemiology and pathogenesis.AIDS Rev. 2003 Oct-Dec;5(4):222-9. AIDS Rev. 2003. PMID: 15012001 Review.
Cited by
-
Exploring the Seasonal Drivers of Varicella Zoster Virus Transmission and Reactivation.Am J Epidemiol. 2021 Sep 1;190(9):1814-1820. doi: 10.1093/aje/kwab073. Am J Epidemiol. 2021. PMID: 33733653 Free PMC article.
-
Distinct roles for extracellular signal-regulated kinase 1 (ERK1) and ERK2 in the structure and production of a primate gammaherpesvirus.J Virol. 2012 Sep;86(18):9721-36. doi: 10.1128/JVI.00695-12. Epub 2012 Jun 27. J Virol. 2012. PMID: 22740395 Free PMC article.
-
Murine Gammaherpesvirus 68 ORF45 Stimulates B2 Retrotransposon and Pre-tRNA Activation in a Manner Dependent on Mitogen-Activated Protein Kinase (MAPK) Signaling.Microbiol Spectr. 2023 Feb 8;11(2):e0017223. doi: 10.1128/spectrum.00172-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 36752632 Free PMC article.
-
Activation of p90 ribosomal S6 kinase by ORF45 of Kaposi's sarcoma-associated herpesvirus and its role in viral lytic replication.J Virol. 2008 Feb;82(4):1838-50. doi: 10.1128/JVI.02119-07. Epub 2007 Dec 5. J Virol. 2008. PMID: 18057234 Free PMC article.
-
Transcriptional and post-transcriptional regulation of viral gene expression in the gamma-herpesvirus Kaposi's sarcoma-associated herpesvirus.Curr Clin Microbiol Rep. 2018 Dec;5(4):219-228. doi: 10.1007/s40588-018-0102-1. Epub 2018 Aug 3. Curr Clin Microbiol Rep. 2018. PMID: 30854283 Free PMC article.
References
-
- Pellett PE, Roizman B. The family herpesviridae: A brief introduction. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, et al., editors. Fields virology. 4th edition. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2381–2398.
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Sugden B. Epstein-Barr virus: A human pathogen inducing lymphoproliferation in vivo and in vitro. Rev Infect Dis. 1982;4:1048–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

