Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T
- PMID: 17360477
- PMCID: PMC1802729
- DOI: 10.1073/pnas.0611665104
Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T
Abstract
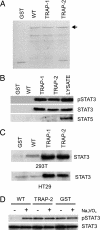
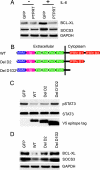
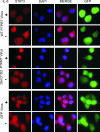
Protein tyrosine phosphatase (PTP) receptor T (PTPRT) is the most frequently mutated PTP in human cancers. However, the cell signaling pathways regulated by PTPRT have not yet been elucidated. Here, we report identification of signal transducer and activator of transcription 3 (STAT3) as a substrate of PTPRT. Phosphorylation of a tyrosine at amino acid Y705 is essential for the function of STAT3, and PTPRT specifically dephosphorylated STAT3 at this position. Accordingly, overexpression of normal PTPRT in colorectal cancer cells reduced the expression of STAT3 target genes. These studies illuminate a mechanism regulating the STAT3 pathway and suggest that this signaling pathway plays an important role in colorectal tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
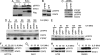
Similar articles
-
Identification and functional characterization of paxillin as a target of protein tyrosine phosphatase receptor T.Proc Natl Acad Sci U S A. 2010 Feb 9;107(6):2592-7. doi: 10.1073/pnas.0914884107. Epub 2010 Jan 21. Proc Natl Acad Sci U S A. 2010. PMID: 20133777 Free PMC article.
-
Frequent promoter hypermethylation of PTPRT increases STAT3 activation and sensitivity to STAT3 inhibition in head and neck cancer.Oncogene. 2016 Mar 3;35(9):1163-9. doi: 10.1038/onc.2015.171. Epub 2015 May 18. Oncogene. 2016. PMID: 25982282 Free PMC article.
-
Activation of signal transducer and activator of transcription 3 through a phosphomimetic serine 727 promotes prostate tumorigenesis independent of tyrosine 705 phosphorylation.Cancer Res. 2008 Oct 1;68(19):7736-41. doi: 10.1158/0008-5472.CAN-08-1125. Cancer Res. 2008. PMID: 18829527 Free PMC article.
-
Protein Tyrosine Phosphatases as Potential Regulators of STAT3 Signaling.Int J Mol Sci. 2018 Sep 11;19(9):2708. doi: 10.3390/ijms19092708. Int J Mol Sci. 2018. PMID: 30208623 Free PMC article. Review.
-
Tumour suppressor function of protein tyrosine phosphatase receptor-T.Biosci Rep. 2011 Oct;31(5):303-7. doi: 10.1042/BSR20100134. Biosci Rep. 2011. PMID: 21517784 Free PMC article. Review.
Cited by
-
Robust small molecule-aided cardiac reprogramming systems selective to cardiac fibroblasts.iScience. 2023 Nov 14;26(12):108466. doi: 10.1016/j.isci.2023.108466. eCollection 2023 Dec 15. iScience. 2023. PMID: 38077137 Free PMC article.
-
Protein tyrosine phosphatases, TC-PTP, SHP1, and SHP2, cooperate in rapid dephosphorylation of Stat3 in keratinocytes following UVB irradiation.PLoS One. 2010 Apr 22;5(4):e10290. doi: 10.1371/journal.pone.0010290. PLoS One. 2010. PMID: 20421975 Free PMC article.
-
STAT3 Role in T-Cell Memory Formation.Int J Mol Sci. 2022 Mar 7;23(5):2878. doi: 10.3390/ijms23052878. Int J Mol Sci. 2022. PMID: 35270020 Free PMC article. Review.
-
Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells.Int J Cancer. 2011 Sep 1;129(5):1053-63. doi: 10.1002/ijc.25764. Epub 2011 Jan 7. Int J Cancer. 2011. PMID: 21710491 Free PMC article.
-
Cross-talk between phospho-STAT3 and PLCγ1 plays a critical role in colorectal tumorigenesis.Mol Cancer Res. 2011 Oct;9(10):1418-28. doi: 10.1158/1541-7786.MCR-11-0147. Epub 2011 Aug 12. Mol Cancer Res. 2011. PMID: 21840932 Free PMC article.
References
-
- Blume-Jensen P, Hunter T. Nature. 2001;411:355–365. - PubMed
-
- Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, Szabo S, Ptak J, Silliman N, Peters BA, van der Heijden MS, et al. Science. 2004;304:1164–1166. - PubMed
-
- Collier LS, Carlson CM, Ravimohan S, Dupuy AJ, Largaespada DA. Nature. 2005;436:272–276. - PubMed
-
- Bowman T, Garcia R, Turkson J, Jove R. Oncogene. 2000;19:2474–2488. - PubMed
-
- Yu H, Jove R. Nat Rev Cancer. 2004;4:97–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

