Immunotherapy with canarypox vaccine and interleukin-2 for HIV-1 infection: termination of a randomized trial
- PMID: 17260026
- PMCID: PMC1783674
- DOI: 10.1371/journal.pctr.0020005
Immunotherapy with canarypox vaccine and interleukin-2 for HIV-1 infection: termination of a randomized trial
Abstract
Objectives: To determine whether immunotherapy of chronic HIV-1 infection can prevent or attenuate viremia upon antiviral discontinuation.
Design: This was a Phase II randomized, partially double blinded, 2x2 factorial study of three steps of 12 wk/step. Step I involved four groups: (1) vaccine placebo, (2) vaccine (ALVAC, vCP1452), (3) placebo + interleukin 2 (IL-2), and (4) vaccine + IL-2. Step II involved a 12-wk diagnostic treatment interruption (DTI). Step III involved an extension of the DTI for an additional 12 wk.
Setting: The Weill-Cornell General Clinical Research Center.
Participants: Chronically infected HIV-1 positive adults with undetectable HIV-1 levels and > 400 CD4+ T cells/microl.
Interventions: An HIV canarypox vaccine (vCP1452) and vaccine placebo, administered every 4 wk for four doses, and low-dose IL-2 administered daily for 12-24 wk.
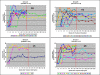
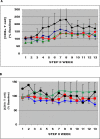
Outcome measures: Primary endpoints: (1) Proportion of participants with undetectable plasma HIV RNA during trial Step II, (2) mean log10 HIV RNA copies/ml ([HIV]) from weeks 21-25, and (3) proportion of individuals eligible for trial Step III.
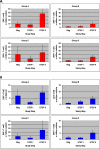
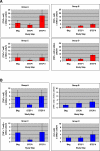
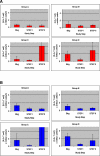
Results: 44 participants were randomized, but 16 withdrew or were withdrawn before completing Step II. As all participants underwent viral relapse in Step II, the study was terminated after 28 participants completed Step II. Among the four groups, there was no difference in mean [HIV] or the proportion of individuals with < log10 4.48 HIV; no difference between the mean [HIV] of the two groups that received ALVAC (n = 17) versus placebo (n = 11); and no significant difference between the mean [HIV] of the two groups that received IL-2 (n = 11) versus placebo (n = 17).
Conclusions: Neither ALVAC (vCP1452) nor low-dose daily IL-2 nor their combination prevented the relapse of viremia upon discontinuation of antiviral therapy.
Conflict of interest statement
Figures


Similar articles
-
Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452).AIDS. 2008 Jul 11;22(11):1313-22. doi: 10.1097/QAD.0b013e3282fdce94. AIDS. 2008. PMID: 18580611 Clinical Trial.
-
Phase I/II randomized trial of safety and immunogenicity of LIPO-5 alone, ALVAC-HIV (vCP1452) alone, and ALVAC-HIV (vCP1452) prime/LIPO-5 boost in healthy, HIV-1-uninfected adult participants.Clin Vaccine Immunol. 2014 Nov;21(11):1589-99. doi: 10.1128/CVI.00450-14. Epub 2014 Sep 24. Clin Vaccine Immunol. 2014. PMID: 25253665 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Early antiviral treatment in outpatients with COVID-19 (FLARE): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Mar 8;22(1):193. doi: 10.1186/s13063-021-05139-2. Trials. 2021. PMID: 33685502 Free PMC article.
-
Antihelminthics in helminth-endemic areas: effects on HIV disease progression.Cochrane Database Syst Rev. 2016 Apr 14;4(4):CD006419. doi: 10.1002/14651858.CD006419.pub4. Cochrane Database Syst Rev. 2016. PMID: 27075622 Free PMC article. Review.
Cited by
-
Safety of low-dose subcutaneous recombinant interleukin-2: systematic review and meta-analysis of randomized controlled trials.Sci Rep. 2019 May 9;9(1):7145. doi: 10.1038/s41598-019-43530-x. Sci Rep. 2019. PMID: 31073219 Free PMC article.
-
Partially randomized, non-blinded trial of DNA and MVA therapeutic vaccines based on hepatitis B virus surface protein for chronic HBV infection.PLoS One. 2011 Feb 15;6(2):e14626. doi: 10.1371/journal.pone.0014626. PLoS One. 2011. PMID: 21347224 Free PMC article. Clinical Trial.
-
PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells.J Clin Invest. 2013 Jun;123(6):2604-15. doi: 10.1172/JCI67008. Epub 2013 May 15. J Clin Invest. 2013. PMID: 23676462 Free PMC article.
-
Smallpox: can we still learn from the journey to eradication?Indian J Med Res. 2013 May;137(5):895-9. Indian J Med Res. 2013. PMID: 23760373 Free PMC article. Review.
-
Role of interleukin-2 in patients with HIV infection.Drugs. 2010 Jun 18;70(9):1115-30. doi: 10.2165/10898620-000000000-00000. Drugs. 2010. PMID: 20518579 Review.
References
-
- Jubault V, Burgard M, Le Corfec E, Costagliola D, Rouziox C, et al. High rebound of plasma and cellular HIV load after discontinuation of triple combination therapy. AIDS. 1998;12:2358–2359. - PubMed
-
- Staszewski S, Miller V, Sabin C, Berger A, Hill AM, et al. Rebound of HIV-1 viral load after suppression to very low levels. AIDS. 1998;12:2360. - PubMed
-
- Smith K. To cure chronic HIV infection, a new strategy is needed. Curr Opin Immunol. 2001;13:617–624. - PubMed
-
- Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

