Epstein-Barr virus BZLF1 gene, a switch from latency to lytic infection, is expressed as an immediate-early gene after primary infection of B lymphocytes
- PMID: 17079287
- PMCID: PMC1797481
- DOI: 10.1128/JVI.01416-06
Epstein-Barr virus BZLF1 gene, a switch from latency to lytic infection, is expressed as an immediate-early gene after primary infection of B lymphocytes
Abstract
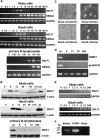
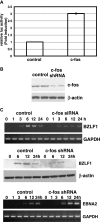
We demonstrate here that the Epstein-Barr virus (EBV) BZLF1 gene, a switch from latent infection to lytic infection, is expressed as early as 1.5 h after EBV infection in Burkitt's lymphoma-derived, EBV-negative Akata and Daudi cells and primary B lymphocytes. Since BZLF1 mRNA is expressed even when the cells are infected with EBV in the presence of anisomycin, an inhibitor of protein synthesis, its expression does not require prerequisite protein synthesis, indicating that BZLF1 is expressed as an immediate-early gene following primary EBV infection of B lymphocytes.
Figures

Similar articles
-
Immune activation suppresses initiation of lytic Epstein-Barr virus infection.Cell Microbiol. 2007 Aug;9(8):2055-69. doi: 10.1111/j.1462-5822.2007.00937.x. Epub 2007 Apr 5. Cell Microbiol. 2007. PMID: 17419714
-
One-step multiplex real-time PCR assay to analyse the latency patterns of Epstein-Barr virus infection.J Virol Methods. 2008 Jan;147(1):26-36. doi: 10.1016/j.jviromet.2007.08.012. Epub 2007 Sep 17. J Virol Methods. 2008. PMID: 17870188
-
A molecular link between malaria and Epstein-Barr virus reactivation.PLoS Pathog. 2007 Jun;3(6):e80. doi: 10.1371/journal.ppat.0030080. PLoS Pathog. 2007. PMID: 17559303 Free PMC article.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
Cited by
-
Phase I clinical trial of valacyclovir and standard of care cyclophosphamide in children with endemic Burkitt lymphoma in Malawi.Clin Lymphoma Myeloma Leuk. 2013 Apr;13(2):112-8. doi: 10.1016/j.clml.2012.11.003. Epub 2012 Dec 20. Clin Lymphoma Myeloma Leuk. 2013. PMID: 23260601 Free PMC article. Clinical Trial.
-
To be or not IIb: a multi-step process for Epstein-Barr virus latency establishment and consequences for B cell tumorigenesis.PLoS Pathog. 2015 Mar 19;11(3):e1004656. doi: 10.1371/journal.ppat.1004656. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25790223 Free PMC article. Review. No abstract available.
-
Viral manipulation of cellular protein conjugation pathways: The SUMO lesson.World J Virol. 2013 May 12;2(2):79-90. doi: 10.5501/wjv.v2.i2.79. World J Virol. 2013. PMID: 24175232 Free PMC article. Review.
-
Noise cancellation: viral fine tuning of the cellular environment for its own genome replication.PLoS Pathog. 2010 Dec 16;6(12):e1001158. doi: 10.1371/journal.ppat.1001158. PLoS Pathog. 2010. PMID: 21187893 Free PMC article. Review.
-
Unexpected patterns of Epstein-Barr virus transcription revealed by a high throughput PCR array for absolute quantification of viral mRNA.Virology. 2015 Jan 1;474:117-30. doi: 10.1016/j.virol.2014.10.030. Epub 2014 Nov 15. Virology. 2015. PMID: 25463610 Free PMC article.
References
-
- Alfieri, C., M. Birkenbach, and E. Kieff. 1991. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology 181:595-608. - PubMed
-
- Allday, M. J., D. H. Crawford, and B. E. Griffin. 1989. Epstein-Barr virus latent gene expression during the initiation of B-cell immortalization. J. Gen. Virol. 70:1755-1764. - PubMed
-
- Chen, H., J. M. Lee, Y. Wang, D. P. Huang, R. F. Ambinder, and S. D. Hayward. 1999. The Epstein-Barr virus latency BamHI-Q promoter is positively regulated by STATs and Zta interference with JAK/STAT activation leads to loss of BamHI-Q promoter activity. Proc. Natl. Acad. Sci. USA 96:9339-9344. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

