Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney
- PMID: 17036046
- PMCID: PMC1630416
- DOI: 10.1038/sj.emboj.7601381
Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney
Abstract
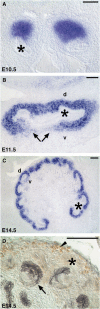
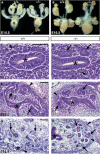
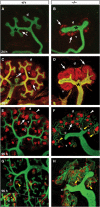
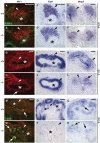
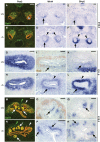
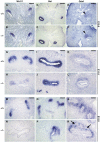
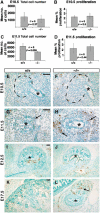
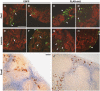
During kidney development and in response to inductive signals, the metanephric mesenchyme aggregates, becomes polarized, and generates much of the epithelia of the nephron. As such, the metanephric mesenchyme is a renal progenitor cell population that must be replenished as epithelial derivatives are continuously generated. The molecular mechanisms that maintain the undifferentiated state of the metanephric mesenchymal precursor cells have not yet been identified. In this paper, we report that functional inactivation of the homeobox gene Six2 results in premature and ectopic differentiation of mesenchymal cells into epithelia and depletion of the progenitor cell population within the metanephric mesenchyme. Failure to renew the mesenchymal cells results in severe renal hypoplasia. Gain of Six2 function in cortical metanephric mesenchymal cells was sufficient to prevent their epithelial differentiation in an organ culture assay. We propose that in the developing kidney, Six2 activity is required for maintaining the mesenchymal progenitor population in an undifferentiated state by opposing the inductive signals emanating from the ureteric bud.
Figures
Similar articles
-
Osr1 acts downstream of and interacts synergistically with Six2 to maintain nephron progenitor cells during kidney organogenesis.Development. 2014 Apr;141(7):1442-52. doi: 10.1242/dev.103283. Epub 2014 Mar 5. Development. 2014. PMID: 24598167 Free PMC article.
-
Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney.Dev Biol. 2008 Dec 1;324(1):88-98. doi: 10.1016/j.ydbio.2008.09.010. Epub 2008 Sep 19. Dev Biol. 2008. PMID: 18835385 Free PMC article.
-
Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development.Cell Stem Cell. 2008 Aug 7;3(2):169-81. doi: 10.1016/j.stem.2008.05.020. Cell Stem Cell. 2008. PMID: 18682239 Free PMC article.
-
Stem cells in the embryonic kidney.Kidney Int. 2008 Apr;73(8):913-7. doi: 10.1038/sj.ki.5002784. Epub 2008 Jan 16. Kidney Int. 2008. PMID: 18200005 Review.
-
Nephron progenitors in the metanephric mesenchyme.Pediatr Nephrol. 2011 Sep;26(9):1463-7. doi: 10.1007/s00467-011-1806-0. Epub 2011 Feb 19. Pediatr Nephrol. 2011. PMID: 21336811 Review.
Cited by
-
Dose-dependent responses to canonical Wnt transcriptional complexes in the regulation of mammalian nephron progenitors.Development. 2024 Sep 15;151(18):dev202279. doi: 10.1242/dev.202279. Epub 2024 Sep 30. Development. 2024. PMID: 39250420 Free PMC article.
-
Transcription factor roles in the local adaptation to temperature in the Andean Spiny Toad Rhinella spinulosa.Sci Rep. 2024 Jul 2;14(1):15158. doi: 10.1038/s41598-024-66127-5. Sci Rep. 2024. PMID: 38956427 Free PMC article.
-
PCP auto count: a novel Fiji/ImageJ plug-in for automated quantification of planar cell polarity and cell counting.Front Cell Dev Biol. 2024 May 17;12:1394031. doi: 10.3389/fcell.2024.1394031. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38827526 Free PMC article.
-
Human pluripotent stem cell-derived kidney organoids: Current progress and challenges.World J Stem Cells. 2024 Feb 26;16(2):114-125. doi: 10.4252/wjsc.v16.i2.114. World J Stem Cells. 2024. PMID: 38455108 Free PMC article. Review.
-
Shared features in ear and kidney development - implications for oto-renal syndromes.Dis Model Mech. 2024 Feb 1;17(2):dmm050447. doi: 10.1242/dmm.050447. Epub 2024 Feb 14. Dis Model Mech. 2024. PMID: 38353121 Free PMC article.
References
-
- Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JB (1993) The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech Dev 40: 85–97 - PubMed
-
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP (2005) Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292 - PubMed
-
- Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR (1998) Differential expression and function of cadherin-6 during renal epithelium development. Development 125: 803–812 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

