Effects of prostratin on Cyclin T1/P-TEFb function and the gene expression profile in primary resting CD4+ T cells
- PMID: 17014716
- PMCID: PMC1599745
- DOI: 10.1186/1742-4690-3-66
Effects of prostratin on Cyclin T1/P-TEFb function and the gene expression profile in primary resting CD4+ T cells
Abstract
Background: The latent reservoir of human immunodeficiency virus type 1 (HIV-1) in resting CD4+ T cells is a major obstacle to the clearance of infection by highly active antiretroviral therapy (HAART). Recent studies have focused on searches for adjuvant therapies to activate this reservoir under conditions of HAART. Prostratin, a non tumor-promoting phorbol ester, is a candidate for such a strategy. Prostratin has been shown to reactivate latent HIV-1 and Tat-mediated transactivation may play an important role in this process. We examined resting CD4+ T cells from healthy donors to determine if prostratin induces Cyclin T1/P-TEFb, a cellular kinase composed of Cyclin T1 and Cyclin-dependent kinase-9 (CDK9) that mediates Tat function. We also examined effects of prostratin on Cyclin T2a, an alternative regulatory subunit for CDK9, and 7SK snRNA and the HEXIM1 protein, two factors that associate with P-TEFb and repress its kinase activity.
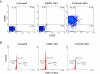
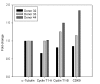
Results: Prostratin up-regulated Cyclin T1 protein expression, modestly induced CDK9 protein expression, and did not affect Cyclin T2a protein expression. Although the kinase activity of CDK9 in vitro was up-regulated by prostratin, we observed a large increase in the association of 7SK snRNA and the HEXIM1 protein with CDK9. Using HIV-1 reporter viruses with and without a functional Tat protein, we found that prostratin stimulation of HIV-1 gene expression appears to require a functional Tat protein. Microarray analyses were performed and several genes related to HIV biology, including APOBEC3B, DEFA1, and S100 calcium-binding protein genes, were found to be regulated by prostratin.
Conclusion: Prostratin induces Cyclin T1 expression and P-TEFb function and this is likely to be involved in prostratin reactivation of latent HIV-1 proviruses. The large increase in association of 7SK and HEXIM1 with P-TEFb following prostratin treatment may reflect a requirement in CD4+ T cells for a precise balance between active and catalytically inactive P-TEFb. Additionally, genes regulated by prostratin were identified that have the potential to regulate HIV-1 replication both positively and negatively.
Figures



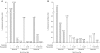
Similar articles
-
Short Communication: The Broad-Spectrum Histone Deacetylase Inhibitors Vorinostat and Panobinostat Activate Latent HIV in CD4(+) T Cells In Part Through Phosphorylation of the T-Loop of the CDK9 Subunit of P-TEFb.AIDS Res Hum Retroviruses. 2016 Feb;32(2):169-73. doi: 10.1089/AID.2015.0347. AIDS Res Hum Retroviruses. 2016. PMID: 26727990 Free PMC article.
-
Regulation of TAK/P-TEFb in CD4+ T lymphocytes and macrophages.Curr HIV Res. 2003 Oct;1(4):395-404. doi: 10.2174/1570162033485159. Curr HIV Res. 2003. PMID: 15049426 Review.
-
Phosphorylation of CDK9 at Ser175 enhances HIV transcription and is a marker of activated P-TEFb in CD4(+) T lymphocytes.PLoS Pathog. 2013;9(5):e1003338. doi: 10.1371/journal.ppat.1003338. Epub 2013 May 2. PLoS Pathog. 2013. PMID: 23658523 Free PMC article. Clinical Trial.
-
Tat competes with HEXIM1 to increase the active pool of P-TEFb for HIV-1 transcription.Nucleic Acids Res. 2007;35(6):2003-12. doi: 10.1093/nar/gkm063. Epub 2007 Mar 6. Nucleic Acids Res. 2007. PMID: 17341462 Free PMC article.
-
The HIV-1 Tat Protein: Mechanism of Action and Target for HIV-1 Cure Strategies.Curr Pharm Des. 2017;23(28):4098-4102. doi: 10.2174/1381612823666170704130635. Curr Pharm Des. 2017. PMID: 28677507 Free PMC article. Review.
Cited by
-
MicroRNA-mediated restriction of HIV-1 in resting CD4+ T cells and monocytes.Viruses. 2012 Sep;4(9):1390-409. doi: 10.3390/v4091390. Epub 2012 Aug 29. Viruses. 2012. PMID: 23170164 Free PMC article. Review.
-
Regulation of cyclin T1 and HIV-1 Replication by microRNAs in resting CD4+ T lymphocytes.J Virol. 2012 Mar;86(6):3244-52. doi: 10.1128/JVI.05065-11. Epub 2011 Dec 28. J Virol. 2012. PMID: 22205749 Free PMC article.
-
Cyclin T1-dependent genes in activated CD4 T and macrophage cell lines appear enriched in HIV-1 co-factors.PLoS One. 2008 Sep 5;3(9):e3146. doi: 10.1371/journal.pone.0003146. PLoS One. 2008. PMID: 18773076 Free PMC article.
-
Non-coding RNAs in the Pathogenesis of Multiple Sclerosis.Front Genet. 2021 Sep 30;12:717922. doi: 10.3389/fgene.2021.717922. eCollection 2021. Front Genet. 2021. PMID: 34659340 Free PMC article. Review.
-
Phosphatase PPM1A regulates phosphorylation of Thr-186 in the Cdk9 T-loop.J Biol Chem. 2008 Nov 28;283(48):33578-84. doi: 10.1074/jbc.M807495200. Epub 2008 Oct 1. J Biol Chem. 2008. PMID: 18829461 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

