Three restricted forms of Epstein-Barr virus latency counteracting apoptosis in c-myc-expressing Burkitt lymphoma cells
- PMID: 17001014
- PMCID: PMC1595454
- DOI: 10.1073/pnas.0509988103
Three restricted forms of Epstein-Barr virus latency counteracting apoptosis in c-myc-expressing Burkitt lymphoma cells
Abstract
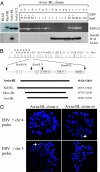
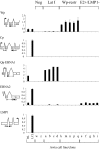
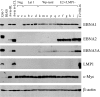
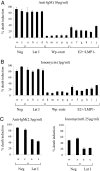
Epstein-Barr virus (EBV), a human herpesvirus, transforms B cell growth in vitro through expressing six virus-coded Epstein-Barr nuclear antigens (EBNAs) and two latent membrane proteins (LMPs). In many EBV-associated tumors, however, viral antigen expression is more restricted, and the aetiological role of the virus is unclear. For example, endemic Burkitt lymphoma (BL) classically presents as a monoclonal, c-myc-translocation-positive tumor in which every cell carries EBV as an EBNA1-only (Latency I) infection; such homogeneity among EBV-positive cells, and the lack of EBV-negative comparators, hampers attempts to understand EBV's role in BL pathogenesis. Here, we describe an endemic BL that was unusually heterogeneous at the single-cell level and, in early passage culture, yielded a range of cellular clones, all with the same c-myc translocation but differing in EBV status. Rare EBV-negative cells were isolated alongside EBV-positive cells displaying one of three forms of restricted latency: (i) conventional Latency I expressing EBNA1 only from a WT virus genome, (ii) Wp-restricted latency expressing EBNAs 1, 3A, 3B, 3C, and -LP only from an EBNA2-deleted genome, and (iii) a previously undescribed EBNA2(+)/LMP1(-) latency in which all six EBNAs are expressed again in the absence of the LMPs. Interclonal comparisons showed that each form of EBV infection was associated with a specific degree of protection from apoptosis. Our work suggests that EBV acts as an antiapoptotic rather than a growth-promoting agent in BL by selecting among three transcriptional programs, all of which, unlike the full virus growth-transforming program, remain compatible with high c-myc expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Epstein-Barr virus nuclear antigen 2 (EBNA2) gene deletion is consistently linked with EBNA3A, -3B, and -3C expression in Burkitt's lymphoma cells and with increased resistance to apoptosis.J Virol. 2005 Aug;79(16):10709-17. doi: 10.1128/JVI.79.16.10709-10717.2005. J Virol. 2005. PMID: 16051863 Free PMC article.
-
Epstein-Barr virus-associated Burkitt lymphomagenesis selects for downregulation of the nuclear antigen EBNA2.Nat Med. 2002 Oct;8(10):1098-104. doi: 10.1038/nm758. Epub 2002 Sep 3. Nat Med. 2002. PMID: 12219084
-
EBNA2-deleted Epstein-Barr virus (EBV) isolate, P3HR1, causes Hodgkin-like lymphomas and diffuse large B cell lymphomas with type II and Wp-restricted latency types in humanized mice.PLoS Pathog. 2020 Jun 15;16(6):e1008590. doi: 10.1371/journal.ppat.1008590. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32542010 Free PMC article.
-
EBNA2 and Its Coactivator EBNA-LP.Curr Top Microbiol Immunol. 2015;391:35-59. doi: 10.1007/978-3-319-22834-1_2. Curr Top Microbiol Immunol. 2015. PMID: 26428371 Review.
-
EBV regulates c-MYC, apoptosis, and tumorigenicity in Burkitt's lymphoma.Curr Top Microbiol Immunol. 2001;258:153-60. doi: 10.1007/978-3-642-56515-1_10. Curr Top Microbiol Immunol. 2001. PMID: 11443860 Review. No abstract available.
Cited by
-
Deregulation of the cell cycle machinery by Epstein-Barr virus nuclear antigen 3C.Future Virol. 2009 Jan;4(1):79-91. doi: 10.2217/17460794.4.1.79. Future Virol. 2009. PMID: 25635182 Free PMC article.
-
Restricted TET2 Expression in Germinal Center Type B Cells Promotes Stringent Epstein-Barr Virus Latency.J Virol. 2017 Feb 14;91(5):e01987-16. doi: 10.1128/JVI.01987-16. Print 2017 Mar 1. J Virol. 2017. PMID: 28003489 Free PMC article.
-
Exploiting the interplay between innate and adaptive immunity to improve immunotherapeutic strategies for Epstein-Barr-virus-driven disorders.Clin Dev Immunol. 2012;2012:931952. doi: 10.1155/2012/931952. Epub 2012 Jan 29. Clin Dev Immunol. 2012. PMID: 22319542 Free PMC article. Review.
-
Methylation status of the Epstein-Barr virus (EBV) BamHI W latent cycle promoter and promoter activity: analysis with novel EBV-positive Burkitt and lymphoblastoid cell lines.J Virol. 2006 Nov;80(21):10700-11. doi: 10.1128/JVI.01204-06. Epub 2006 Aug 18. J Virol. 2006. PMID: 16920819 Free PMC article.
-
Quantitative studies of Epstein-Barr virus-encoded microRNAs provide novel insights into their regulation.J Virol. 2011 Jan;85(2):996-1010. doi: 10.1128/JVI.01528-10. Epub 2010 Nov 10. J Virol. 2011. PMID: 21068248 Free PMC article.
References
-
- Kuppers R. Nat Rev Immunol. 2003;3:801–812. - PubMed
-
- Rickinson AB, Kieff E. In: Fields Virology. Knipe DM, Howley PM, editors. Vol II. Philadelphia: Lippincott; 2001. pp. 2575–2627.
-
- Kieff E, Rickinson A. In: Fields Virology. Knipe DM, Howley PM, editors. Vol II. Philadelphia: Lippincott; 2001. pp. 2511–2573.
-
- Magrath I. Adv Cancer Res. 1990;55:133–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

