Latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors
- PMID: 16956952
- PMCID: PMC1641760
- DOI: 10.1128/JVI.00673-06
Latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors
Abstract
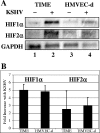
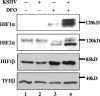
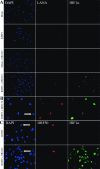
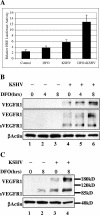
Kaposi's sarcoma-associated herpesvirus (KSHV or HHV-8) is the etiological agent of Kaposi's sarcoma, a highly vascularized, endothelial-derived tumor. A direct role for KSHV-mediated induction of angiogenesis has been proposed based upon the nature of the neoplasia and various KSHV gene overexpression and infection model systems. We have found that KSHV infection of endothelial cells induces mRNA of hypoxia-induced factor 1alpha (HIF1alpha) and HIF2alpha, two homologous alpha subunits of the heterodimeric transcription factor HIF. HIF is a master regulator of both developmental and pathological angiogenesis, composed of an oxygen-sensitive alpha subunit and a constitutively expressed beta subunit. HIF is classically activated posttranscriptionally with hypoxia, leading to increased protein stability of HIF1alpha and/or HIF2alpha. However, we demonstrate that both alpha subunits are up-regulated at the transcript level by KSHV infection. The transcriptional activation of HIF leads to a functional increase in HIF activity under normoxic conditions, as demonstrated by both luciferase reporter assay and the increased expression of vascular endothelial growth factor receptor 1 (VEGFR1), an HIF-responsive gene. KSHV infection synergizes with hypoxia mimics and induces higher expression levels of HIF1alpha and HIF2alpha protein, and HIF1alpha is increased in a significant proportion of the latently infected endothelial cells. Src family kinases are required for the activation of HIF and the downstream gene VEGFR1 by KSHV. We also show that KS lesions, in vivo, express elevated levels of HIF1alpha and HIF2alpha proteins. Thus, KSHV stimulates the HIF pathway via transcriptional up-regulation of both HIF alphas, and this activation may play a role in KS formation, localization, and progression.
Figures




Similar articles
-
The Kaposi's Sarcoma-Associated Herpesvirus ORF34 Protein Interacts and Stabilizes HIF-2α via Binding to the HIF-2α bHLH and PAS Domains.J Virol. 2019 Aug 13;93(17):e00764-19. doi: 10.1128/JVI.00764-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31189709 Free PMC article.
-
Inhibition of KAP1 enhances hypoxia-induced Kaposi's sarcoma-associated herpesvirus reactivation through RBP-Jκ.J Virol. 2014 Jun;88(12):6873-84. doi: 10.1128/JVI.00283-14. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696491 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Infection Induces the Expression of Neuroendocrine Genes in Endothelial Cells.J Virol. 2020 Mar 31;94(8):e01692-19. doi: 10.1128/JVI.01692-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969437 Free PMC article.
-
The contribution of systems biology and reverse genetics to the understanding of Kaposi's sarcoma-associated herpesvirus pathogenesis in endothelial cells.Thromb Haemost. 2009 Dec;102(6):1117-34. doi: 10.1160/TH09-07-0472. Thromb Haemost. 2009. PMID: 19967142 Review.
-
Activation of cellular metabolism during latent Kaposi's Sarcoma herpesvirus infection.Curr Opin Virol. 2016 Aug;19:45-9. doi: 10.1016/j.coviro.2016.06.012. Epub 2016 Jul 18. Curr Opin Virol. 2016. PMID: 27434732 Free PMC article. Review.
Cited by
-
An Oncogenic Virus Promotes Cell Survival and Cellular Transformation by Suppressing Glycolysis.PLoS Pathog. 2016 May 17;12(5):e1005648. doi: 10.1371/journal.ppat.1005648. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27187079 Free PMC article.
-
A potential alpha-helix motif in the amino terminus of LANA encoded by Kaposi's sarcoma-associated herpesvirus is critical for nuclear accumulation of HIF-1alpha in normoxia.J Virol. 2007 Oct;81(19):10413-23. doi: 10.1128/JVI.00611-07. Epub 2007 Jul 18. J Virol. 2007. PMID: 17634230 Free PMC article.
-
DLX1008 (brolucizumab), a single-chain anti-VEGF-A antibody fragment with low picomolar affinity, leads to tumor involution in an in vivo model of Kaposi Sarcoma.PLoS One. 2020 May 14;15(5):e0233116. doi: 10.1371/journal.pone.0233116. eCollection 2020. PLoS One. 2020. PMID: 32407363 Free PMC article.
-
Amplification of the angiogenic signal through the activation of the TSC/mTOR/HIF axis by the KSHV vGPCR in Kaposi's sarcoma.PLoS One. 2011 Apr 29;6(4):e19103. doi: 10.1371/journal.pone.0019103. PLoS One. 2011. PMID: 21559457 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus confers a survival advantage to endothelial cells.Cancer Res. 2008 Jun 15;68(12):4640-8. doi: 10.1158/0008-5472.CAN-07-5988. Cancer Res. 2008. PMID: 18559509 Free PMC article.
References
-
- Bedogni, B., S. M. Welford, D. S. Cassarino, B. J. Nickoloff, A. J. Giaccia, and M. B. Powell. 2005. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cells 8:443-454. - PubMed
-
- Blackbourn, D. J., S. Fujimura, T. Kutzkey, and J. A. Levy. 2000. Induction of human herpesvirus-8 gene expression by recombinant interferon gamma. AIDS 14:98-99. - PubMed
-
- Bosco, M. C., M. Puppo, S. Pastorino, Z. Mi, G. Melillo, S. Massazza, A. Rapisarda, and L. Varesio. 2004. Hypoxia selectively inhibits monocyte chemoattractant protein-1 production by macrophages. J. Immunol. 172:1681-1690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

