JAK inhibitors AG-490 and WHI-P154 decrease IFN-gamma-induced iNOS expression and NO production in macrophages
- PMID: 16883061
- PMCID: PMC1592588
- DOI: 10.1155/MI/2006/16161
JAK inhibitors AG-490 and WHI-P154 decrease IFN-gamma-induced iNOS expression and NO production in macrophages
Abstract
In inflammation, inducible nitric oxide synthase (iNOS) produces nitric oxide (NO), which modulates inflammatory processes. We investigated the effects of Janus kinase (JAK) inhibitors, AG-490 and WHI-P154, on iNOS expression and NO production in J774 murine macrophages stimulated with interferon-gamma (IFN-gamma). JAK inhibitors AG-490 and WHI-P154 decreased IFN-gamma-induced nuclear levels of signal transducer and activator of transcription 1alpha (STAT1alpha). JAK inhibitors AG-490 and WHI-P154 decreased also iNOS protein and mRNA expression and NO production in a concentration-dependent manner. Neither of the JAK inhibitors affected the decay of iNOS mRNA when determined by actinomycin D assay. Our results suggest that the inhibition of JAK-STAT1-pathway by AG-490 or WHI-P154 leads to the attenuation of iNOS expression and NO production in IFN-gamma-stimulated macrophages.
Figures


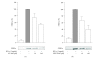
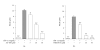



Similar articles
-
Janus kinase 3 inhibitor WHI-P154 in macrophages activated by bacterial endotoxin: differential effects on the expression of iNOS, COX-2 and TNF-alpha.Int Immunopharmacol. 2008 Jan;8(1):100-8. doi: 10.1016/j.intimp.2007.10.016. Epub 2007 Nov 20. Int Immunopharmacol. 2008. PMID: 18068105
-
Inhibition of inducible nitric-oxide synthase expression by (5R)-5-hydroxytriptolide in interferon-gamma- and bacterial lipopolysaccharide-stimulated macrophages.J Pharmacol Exp Ther. 2006 Jan;316(1):121-8. doi: 10.1124/jpet.105.093179. Epub 2005 Sep 15. J Pharmacol Exp Ther. 2006. PMID: 16166270
-
Inhibition of classical PKC isoenzymes downregulates STAT1 activation and iNOS expression in LPS-treated murine J774 macrophages.Br J Pharmacol. 2006 Apr;147(7):790-9. doi: 10.1038/sj.bjp.0706672. Br J Pharmacol. 2006. PMID: 16432499 Free PMC article.
-
The role of NF-kappaB, IRF-1, and STAT-1alpha transcription factors in the iNOS gene induction by gliadin and IFN-gamma in RAW 264.7 macrophages.J Mol Med (Berl). 2006 Jan;84(1):65-74. doi: 10.1007/s00109-005-0713-x. Epub 2005 Nov 12. J Mol Med (Berl). 2006. PMID: 16284791
-
Lipoteichoic acid from Lactobacillus plantarum induces nitric oxide production in the presence of interferon-γ in murine macrophages.Mol Immunol. 2011 Sep;48(15-16):2170-7. doi: 10.1016/j.molimm.2011.07.009. Epub 2011 Aug 10. Mol Immunol. 2011. PMID: 21835472
Cited by
-
JAK-STAT signaling pathways are activated in the brain following reovirus infection.J Neurovirol. 2007 Aug;13(4):373-83. doi: 10.1080/13550280701344983. J Neurovirol. 2007. PMID: 17849321 Free PMC article.
-
Tyrosine kinase inhibitor tyrphostin AG490 retards chronic joint inflammation in mice.Inflammation. 2014 Aug;37(4):995-1005. doi: 10.1007/s10753-014-9820-6. Inflammation. 2014. PMID: 24473905
-
Effects of levo- and dextrosimendan on NF-kappaB-mediated transcription, iNOS expression and NO production in response to inflammatory stimuli.Br J Pharmacol. 2008 Nov;155(6):884-95. doi: 10.1038/bjp.2008.328. Epub 2008 Aug 18. Br J Pharmacol. 2008. PMID: 19002103 Free PMC article.
-
Eriocalyxin B Inhibits STAT3 Signaling by Covalently Targeting STAT3 and Blocking Phosphorylation and Activation of STAT3.PLoS One. 2015 May 26;10(5):e0128406. doi: 10.1371/journal.pone.0128406. eCollection 2015. PLoS One. 2015. PMID: 26010889 Free PMC article.
-
Janus kinase-3 dependent inflammatory responses in allergic asthma.Int Immunopharmacol. 2010 Aug;10(8):829-36. doi: 10.1016/j.intimp.2010.04.014. Epub 2010 Apr 27. Int Immunopharmacol. 2010. PMID: 20430118 Free PMC article. Review.
References
-
- Gao J, Morrison DC, Parmely TJ, Russell SW, Murphy WJ. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. The Journal of Biological Chemistry. 1997;272(2):1226–1230. - PubMed
-
- Gatto L, Berlato C, Poli V, et al. Analysis of SOCS-3 promoter responses to interferon gamma. The Journal of Biological Chemistry. 2004;279(14):13746–13754. - PubMed
-
- Geller DA, Billiar TR. Molecular biology of nitric oxide synthases. Cancer and Metastasis Reviews. 1998;17(1):7–23. - PubMed
-
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry. 1982;126(1):131–138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

