Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4
- PMID: 16710025
- PMCID: PMC2140223
- DOI: 10.1200/JCO.2005.04.5716
Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4
Abstract
Purpose: Cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) is an inhibitory receptor on T cells. Knocking out CTLA4 in mice causes lethal lymphoproliferation, and polymorphisms in human CTLA4 are associated with autoimmune disease. Trials of the anti-CTLA4 antibody ipilimumab (MDX-010) have resulted in durable cancer regression and immune-mediated toxicities. A report on the diagnosis, pathology, treatment, clinical outcome, and significance of the immune-mediated enterocolitis seen with ipilimumab is presented.
Patients and methods: We treated 198 patients with metastatic melanoma (MM) or renal cell carcinoma (RCC) with ipilimumab.
Results: The overall objective tumor response rate was 14%. We observed several immune mediated toxicities including dermatitis, enterocolitis, hypophysitis, uveitis, hepatitis, and nephritis. Enterocolitis, defined by grade 3/4 clinical presentation and/or biopsy documentation, was the most common major toxicity (21% of patients). It presented with diarrhea, and biopsies showed both neutrophilic and lymphocytic inflammation. Most patients who developed enterocolitis responded to high-dose systemic corticosteroids. There was no evidence that steroid administration affected tumor responses. Five patients developed perforation or required colectomy. Four other patients with steroid-refractory enterocolitis appeared to respond promptly to tumor necrosis factor alpha blockade with infliximab. Objective tumor response rates in patients with enterocolitis were 36% for MM and 35% for RCC, compared with 11% and 2% in patients without enterocolitis, respectively (P = .0065 for MM and P = .0016 for RCC).
Conclusion: CTLA4 seems to be a significant component of tolerance to tumor and in protection against immune mediated enterocolitis and these phenomena are significantly associated in cancer patients.
Figures
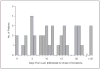
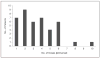

Comment in
-
Is tumor immunity the same thing as autoimmunity? Implications for cancer immunotherapy.J Clin Oncol. 2006 May 20;24(15):2230-2. doi: 10.1200/JCO.2006.05.6952. J Clin Oncol. 2006. PMID: 16710020 No abstract available.
-
Does CTLA4 influence the suppressive effect of CD25+CD4+ regulatory T cells?J Clin Oncol. 2006 Dec 1;24(34):5469-70; author reply 5470-1. doi: 10.1200/JCO.2006.07.5515. J Clin Oncol. 2006. PMID: 17135653 No abstract available.
Similar articles
-
Colonic ulcerations may predict steroid-refractory course in patients with ipilimumab-mediated enterocolitis.World J Gastroenterol. 2017 Mar 21;23(11):2023-2028. doi: 10.3748/wjg.v23.i11.2023. World J Gastroenterol. 2017. PMID: 28373768 Free PMC article.
-
CTLA-4 blockade with monoclonal antibodies in patients with metastatic cancer: surgical issues.Ann Surg Oncol. 2008 Nov;15(11):3014-21. doi: 10.1245/s10434-008-0104-y. Epub 2008 Aug 21. Ann Surg Oncol. 2008. PMID: 18716842 Review.
-
Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab.Dig Dis Sci. 2009 Nov;54(11):2538-40. doi: 10.1007/s10620-008-0641-z. Epub 2008 Dec 23. Dig Dis Sci. 2009. PMID: 19104936 Clinical Trial.
-
Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis.J Immunother. 2007 Nov-Dec;30(8):825-30. doi: 10.1097/CJI.0b013e318156e47e. J Immunother. 2007. PMID: 18049334 Free PMC article. Clinical Trial.
-
The role of the CTLA4 blockade in the treatment of malignant melanoma.Cancer Invest. 2007 Oct;25(7):613-31. doi: 10.1080/07357900701522315. Cancer Invest. 2007. PMID: 18027152 Review.
Cited by
-
Toxicity in the era of immune checkpoint inhibitor therapy.Front Immunol. 2024 Aug 23;15:1447021. doi: 10.3389/fimmu.2024.1447021. eCollection 2024. Front Immunol. 2024. PMID: 39247203 Free PMC article. Review.
-
Human organoids with an autologous tissue-resident immune compartment.Nature. 2024 Sep;633(8028):165-173. doi: 10.1038/s41586-024-07791-5. Epub 2024 Aug 14. Nature. 2024. PMID: 39143209 Free PMC article.
-
Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors and CAR-T Cell Therapy: A Comprehensive Imaging-Based Review.Cancers (Basel). 2024 Jul 19;16(14):2585. doi: 10.3390/cancers16142585. Cancers (Basel). 2024. PMID: 39061225 Free PMC article. Review.
-
The Development of Persistent Gastrointestinal Symptoms in Patients With Melanoma Who Have Had an Immune Checkpoint Inhibitor-Related Gastrointestinal Toxicity.Clin Transl Gastroenterol. 2024 Aug 1;15(8):e00746. doi: 10.14309/ctg.0000000000000746. Clin Transl Gastroenterol. 2024. PMID: 38995215 Free PMC article.
-
Outcomes of Budesonide as a Treatment Option for Immune Checkpoint Inhibitor-Related Colitis in Patients with Cancer.Cancers (Basel). 2024 May 18;16(10):1919. doi: 10.3390/cancers16101919. Cancers (Basel). 2024. PMID: 38791997 Free PMC article.
References
-
- Brunet JF, Dosseto M, Denizot F, et al. The inducible cytotoxic T-lymphocyte-associated gene transcript CTLA-1 sequence and gene localization to mouse chromosome 14. Nature. 1986;322:268–271. - PubMed
-
- Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. - PubMed
-
- Chambers CA, Krummel MF, Boitel B, et al. The role of CTLA-4 in the regulation and initiation of T-cell responses. Immunol Rev. 1996;153:27–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

