Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels
- PMID: 16670769
- PMCID: PMC1451207
- DOI: 10.1172/JCI27100
Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels
Abstract
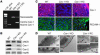
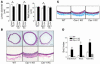
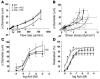
Caveolae in endothelial cells have been implicated as plasma membrane microdomains that sense or transduce hemodynamic changes into biochemical signals that regulate vascular function. Therefore we compared long- and short-term flow-mediated mechanotransduction in vessels from WT mice, caveolin-1 knockout (Cav-1 KO) mice, and Cav-1 KO mice reconstituted with a transgene expressing Cav-1 specifically in endothelial cells (Cav-1 RC mice). Arterial remodeling during chronic changes in flow and shear stress were initially examined in these mice. Ligation of the left external carotid for 14 days to lower blood flow in the common carotid artery reduced the lumen diameter of carotid arteries from WT and Cav-1 RC mice. In Cav-1 KO mice, the decrease in blood flow did not reduce the lumen diameter but paradoxically increased wall thickness and cellular proliferation. In addition, in isolated pressurized carotid arteries, flow-mediated dilation was markedly reduced in Cav-1 KO arteries compared with those of WT mice. This impairment in response to flow was rescued by reconstituting Cav-1 into the endothelium. In conclusion, these results showed that endothelial Cav-1 and caveolae are necessary for both rapid and long-term mechanotransduction in intact blood vessels.
Figures
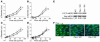
Comment in
-
Role of caveolin-1 in the regulation of the vascular shear stress response.J Clin Invest. 2006 May;116(5):1222-5. doi: 10.1172/JCI28509. J Clin Invest. 2006. PMID: 16670766 Free PMC article.
Similar articles
-
Role of caveolin-1 in the regulation of the vascular shear stress response.J Clin Invest. 2006 May;116(5):1222-5. doi: 10.1172/JCI28509. J Clin Invest. 2006. PMID: 16670766 Free PMC article.
-
Stimulation of Caveolin-1 Signaling Improves Arteriovenous Fistula Patency.Arterioscler Thromb Vasc Biol. 2019 Apr;39(4):754-764. doi: 10.1161/ATVBAHA.119.312417. Arterioscler Thromb Vasc Biol. 2019. PMID: 30786746 Free PMC article.
-
Role of caveolae in shear stress-mediated endothelium-dependent dilation in coronary arteries.Cardiovasc Res. 2013 Oct 1;100(1):151-9. doi: 10.1093/cvr/cvt157. Epub 2013 Jun 19. Cardiovasc Res. 2013. PMID: 23787000 Free PMC article.
-
Caveolae and caveolin-1: novel potential targets for the treatment of cardiovascular disease.Curr Pharm Des. 2007;13(17):1761-9. doi: 10.2174/138161207780831202. Curr Pharm Des. 2007. PMID: 17584106 Review.
-
Caveolin-1: a critical regulator of lung injury.Am J Physiol Lung Cell Mol Physiol. 2011 Feb;300(2):L151-60. doi: 10.1152/ajplung.00170.2010. Epub 2010 Nov 19. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21097526 Free PMC article. Review.
Cited by
-
Phosphocaveolin-1 is a mechanotransducer that induces caveola biogenesis via Egr1 transcriptional regulation.J Cell Biol. 2012 Oct 29;199(3):425-35. doi: 10.1083/jcb.201207089. Epub 2012 Oct 22. J Cell Biol. 2012. PMID: 23091071 Free PMC article.
-
Caveolae as plasma membrane sensors, protectors and organizers.Nat Rev Mol Cell Biol. 2013 Feb;14(2):98-112. doi: 10.1038/nrm3512. Nat Rev Mol Cell Biol. 2013. PMID: 23340574 Review.
-
Caveolae as organizers of pharmacologically relevant signal transduction molecules.Annu Rev Pharmacol Toxicol. 2008;48:359-91. doi: 10.1146/annurev.pharmtox.48.121506.124841. Annu Rev Pharmacol Toxicol. 2008. PMID: 17914930 Free PMC article. Review.
-
Endothelial NOS: perspective and recent developments.Br J Pharmacol. 2019 Jan;176(2):189-196. doi: 10.1111/bph.14522. Epub 2018 Dec 9. Br J Pharmacol. 2019. PMID: 30341769 Free PMC article. Review.
-
Lipid droplet analysis in caveolin-deficient adipocytes: alterations in surface phospholipid composition and maturation defects.J Lipid Res. 2010 May;51(5):945-56. doi: 10.1194/jlr.M001016. Epub 2009 Nov 10. J Lipid Res. 2010. PMID: 19965594 Free PMC article.
References
-
- Drab M., et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. - PubMed
-
- Razani B., et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J. Biol. Chem. 2001;276:38121–38138. - PubMed
-
- Gratton J.P., Bernatchez P., Sessa W.C. Caveolae and caveolins in the cardiovascular system. . Circ. Res. 2004;94:1408–1417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL070295/HL/NHLBI NIH HHS/United States
- R01 HL61371/HL/NHLBI NIH HHS/United States
- R01 HL64793/HL/NHLBI NIH HHS/United States
- R01 HL064793/HL/NHLBI NIH HHS/United States
- R01 HL065418-04/HL/NHLBI NIH HHS/United States
- R01 HL065418/HL/NHLBI NIH HHS/United States
- R01 HL057665/HL/NHLBI NIH HHS/United States
- R01 HL083249/HL/NHLBI NIH HHS/United States
- R01 HL083249-01/HL/NHLBI NIH HHS/United States
- R01 HL061371/HL/NHLBI NIH HHS/United States
- P01 HL70295/HL/NHLBI NIH HHS/United States
- R01 HL57665/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

