Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells
- PMID: 16606841
- PMCID: PMC1459019
- DOI: 10.1073/pnas.0510512103
Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells
Abstract
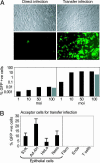
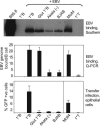
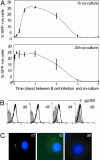
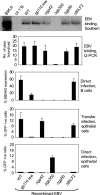
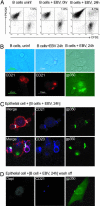
Epstein-Barr virus (EBV), an orally transmitted herpesvirus, efficiently targets B lymphocytes through binding of the viral envelope glycoprotein gp350 to the complement receptor CD21. How the virus accesses epithelial cells is less well understood, because such cells are largely resistant to infection with cell-free virus in vitro. Here, we show that, after binding to primary B cells, most Epstein-Barr virions are not internalized but remain on the B cell surface and from there can transfer efficiently to CD21-negative epithelial cells, increasing epithelial infection by 10(3)- to 10(4)-fold compared with cell-free virus. Transfer infection is associated with the formation of B cell-epithelial conjugates with gp350/CD21 complexes focused at the intercellular synapse; transfer involves the gp85 and gp110 viral glycoproteins but is independent of gp42, the HLA class II ligand that is essential for B cell entry. Therefore, through efficient binding to the B cell surface, EBV has developed a means of simultaneously accessing both lymphoid and epithelial compartments; in particular, infection of pharyngeal epithelium by orally transmitted virus becomes independent of initial virus replication in the B cell system.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Comment in
-
The infectious kiss: newly infected B cells deliver Epstein-Barr virus to epithelial cells.Proc Natl Acad Sci U S A. 2006 May 9;103(19):7201-2. doi: 10.1073/pnas.0602077103. Epub 2006 May 1. Proc Natl Acad Sci U S A. 2006. PMID: 16651525 Free PMC article. No abstract available.
Similar articles
-
Epstein-Barr virus infection of polarized epithelial cells via the basolateral surface by memory B cell-mediated transfer infection.PLoS Pathog. 2011 May;7(5):e1001338. doi: 10.1371/journal.ppat.1001338. Epub 2011 May 5. PLoS Pathog. 2011. PMID: 21573183 Free PMC article.
-
Epstein-Barr virus shed in saliva is high in B-cell-tropic glycoprotein gp42.J Virol. 2006 Jul;80(14):7281-3. doi: 10.1128/JVI.00497-06. J Virol. 2006. PMID: 16809335 Free PMC article.
-
CD21 (Complement Receptor 2) Is the Receptor for Epstein-Barr Virus Entry into T Cells.J Virol. 2020 May 18;94(11):e00428-20. doi: 10.1128/JVI.00428-20. Print 2020 May 18. J Virol. 2020. PMID: 32238579 Free PMC article.
-
[The entry of Epstein-Barr virus into B lymphocytes and epithelial cells during infection].Bing Du Xue Bao. 2014 Jul;30(4):476-82. Bing Du Xue Bao. 2014. PMID: 25272606 Review. Chinese.
-
Two Epstein-Barr virus glycoprotein complexes.Curr Top Microbiol Immunol. 2001;258:51-64. doi: 10.1007/978-3-642-56515-1_4. Curr Top Microbiol Immunol. 2001. PMID: 11443867 Review. No abstract available.
Cited by
-
The Mechanism of PEDV-Carrying CD3+ T Cells Migrate into the Intestinal Mucosa of Neonatal Piglets.Viruses. 2021 Mar 12;13(3):469. doi: 10.3390/v13030469. Viruses. 2021. PMID: 33809123 Free PMC article.
-
The EBV-DNA Can be Used as a Diagnostic and Follow-up Parameter of the Rhinopharyngeal Tumors in the Non-Endemic Population of the Western Sicily.Indian J Otolaryngol Head Neck Surg. 2019 Sep;71(3):396-400. doi: 10.1007/s12070-018-1427-z. Epub 2018 Jun 20. Indian J Otolaryngol Head Neck Surg. 2019. PMID: 31559210 Free PMC article.
-
Immunogenic particles with a broad antigenic spectrum stimulate cytolytic T cells and offer increased protection against EBV infection ex vivo and in mice.PLoS Pathog. 2018 Dec 6;14(12):e1007464. doi: 10.1371/journal.ppat.1007464. eCollection 2018 Dec. PLoS Pathog. 2018. PMID: 30521644 Free PMC article.
-
Meta-analysis of nasopharyngeal carcinoma microarray data explores mechanism of EBV-regulated neoplastic transformation.BMC Genomics. 2008 Jul 7;9:322. doi: 10.1186/1471-2164-9-322. BMC Genomics. 2008. PMID: 18605998 Free PMC article.
-
Epstein-Barr virus induces morphological and molecular changes in thyroid neoplastic cells.Endocrine. 2020 Aug;69(2):321-330. doi: 10.1007/s12020-020-02253-0. Epub 2020 Mar 12. Endocrine. 2020. PMID: 32166585
References
-
- Oda T., Imai S., Chiba S., Takada K. Virology. 2000;276:52–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

