KSHV/HHV-8 infection of human hematopoietic progenitor (CD34+) cells: persistence of infection during hematopoiesis in vitro and in vivo
- PMID: 16543476
- PMCID: PMC1895828
- DOI: 10.1182/blood-2005-04-1697
KSHV/HHV-8 infection of human hematopoietic progenitor (CD34+) cells: persistence of infection during hematopoiesis in vitro and in vivo
Abstract
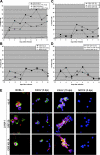
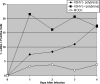
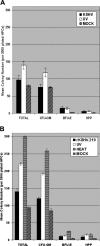
The cellular reservoir for Kaposi sarcoma-associated herpesvirus (KSHV) infection in the hematopoietic compartment and mechanisms governing latent infection and reactivation remain undefined. To determine susceptibility of human CD34+ hematopoietic progenitor cells (HPCs) to infection with KSHV, purified HPCs were exposed to KSHV, and cells were differentiated in vitro and in vivo. Clonogenic colony-forming activity was significantly suppressed in KSHV-infected CD34+ cells, and viral DNA was predominantly localized to granulocyte-macrophage colonies differentiated in vitro. rKSHV.219 is a recombinant KSHV construct that expresses green fluorescent protein from a cellular promoter active during latency and red fluorescent protein from a viral lytic promoter. Infection of CD34+ HPCs with rKSHV.219 showed similar patterns of infection, persistence, and hematopoietic suppression in vitro in comparison with KSHV. rKSHV.219 infection was detected in human CD14+ and CD19+ cells recovered from NOD/SCID mouse bone marrow and spleen following reconstitution with rKSHV.219-infected CD34+ HPCs. These results suggest that rKSHV.219 establishes persistent infection in NOD/SCID mice and that virus may be disseminated following differentiation of infected HPCs into the B-cell and monocyte lineages. CD34+ HPCs may be a reservoir for KSHV infection and may provide a continuous source of virally infected cells in vivo.
Figures


Similar articles
-
Human Cytomegalovirus US28 Ligand Binding Activity Is Required for Latency in CD34+ Hematopoietic Progenitor Cells and Humanized NSG Mice.mBio. 2019 Aug 20;10(4):e01889-19. doi: 10.1128/mBio.01889-19. mBio. 2019. PMID: 31431555 Free PMC article.
-
Human Cytomegalovirus Requires Epidermal Growth Factor Receptor Signaling To Enter and Initiate the Early Steps in the Establishment of Latency in CD34+ Human Progenitor Cells.J Virol. 2017 Feb 14;91(5):e01206-16. doi: 10.1128/JVI.01206-16. Print 2017 Mar 1. J Virol. 2017. PMID: 27974567 Free PMC article.
-
CD34+ Hematopoietic Progenitor Cell Subsets Exhibit Differential Ability To Maintain Human Cytomegalovirus Latency and Persistence.J Virol. 2021 Jan 13;95(3):e02105-20. doi: 10.1128/JVI.02105-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177198 Free PMC article.
-
Viral latent proteins as targets for Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) induced lymphoma.Curr Drug Targets Infect Disord. 2003 Jun;3(2):129-35. doi: 10.2174/1568005033481150. Curr Drug Targets Infect Disord. 2003. PMID: 12769790 Review.
-
Hematopoietic stem cells and betaherpesvirus latency.Front Cell Infect Microbiol. 2023 Jun 6;13:1189805. doi: 10.3389/fcimb.2023.1189805. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37346032 Free PMC article. Review.
Cited by
-
Latent Kaposi's sarcoma-associated herpesvirus infection of monocytes downregulates expression of adaptive immune response costimulatory receptors and proinflammatory cytokines.J Virol. 2012 Apr;86(7):3916-23. doi: 10.1128/JVI.06437-11. Epub 2012 Jan 25. J Virol. 2012. PMID: 22278234 Free PMC article.
-
Kaposi sarcoma-associated herpesvirus degrades cellular Toll-interleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF).J Biol Chem. 2011 Mar 11;286(10):7865-7872. doi: 10.1074/jbc.M110.191452. Epub 2011 Jan 6. J Biol Chem. 2011. PMID: 21212282 Free PMC article.
-
Efficient lytic induction of Kaposi's sarcoma-associated herpesvirus (KSHV) by the anthracyclines.Oncotarget. 2014 Sep 30;5(18):8515-27. doi: 10.18632/oncotarget.2335. Oncotarget. 2014. PMID: 25237786 Free PMC article.
-
The modulation of apoptosis by oncogenic viruses.Virol J. 2013 Jun 6;10:182. doi: 10.1186/1743-422X-10-182. Virol J. 2013. PMID: 23741982 Free PMC article. Review.
-
Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy.J Clin Invest. 2016 Sep 1;126(9):3165-75. doi: 10.1172/JCI84418. Epub 2016 Sep 1. J Clin Invest. 2016. PMID: 27584730 Free PMC article. Review.
References
-
- Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266: 1865-1869. - PubMed
-
- Ganem D. Human herpesvirus 8 and its role in the genesis of Kaposi's sarcoma. Curr Clin Top Infect Dis. 1998;18: 237-251. - PubMed
-
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342: 1027-1038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

