Gene expression patterns in dendritic cells infected with measles virus compared with other pathogens
- PMID: 16492729
- PMCID: PMC1413941
- DOI: 10.1073/pnas.0511345103
Gene expression patterns in dendritic cells infected with measles virus compared with other pathogens
Abstract
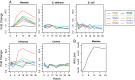
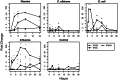
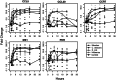
Gene expression patterns supply insight into complex biological networks that provide the organization in which viruses and host cells interact. Measles virus (MV) is an important human pathogen that induces transient immunosuppression followed by life-long immunity in infected individuals. Dendritic cells (DCs) are potent antigen-presenting cells that initiate the immune response to pathogens and are postulated to play a role in MV-induced immunosuppression. To better understand the interaction of MV with DCs, we examined the gene expression changes that occur over the first 24 h after infection and compared these changes to those induced by other viral, bacterial, and fungal pathogens. There were 1,553 significantly regulated genes with nearly 60% of them down-regulated. MV-infected DCs up-regulated a core of genes associated with maturation of antigen-presenting function and migration to lymph nodes but also included genes for IFN-regulatory factors 1 and 7, 2'5' oligoadenylate synthetase, Mx, and TNF superfamily proteins 2, 7, 9, and 10 (TNF-related apoptosis-inducing ligand). MV induced genes for IFNs, ILs, chemokines, antiviral proteins, histones, and metallothioneins, many of which were also induced by influenza virus, whereas genes for protein synthesis and oxidative phosphorylation were down-regulated. Unique to MV were the induction of genes for a broad array of IFN-alphas and the failure to up-regulate dsRNA-dependent protein kinase. These results provide a modular view of common and unique DC responses after infection and suggest mechanisms by which MV may modulate the immune response.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
Measles virus and dendritic cell functions: how specific response cohabits with immunosuppression.Curr Top Microbiol Immunol. 2003;276:103-23. doi: 10.1007/978-3-662-06508-2_5. Curr Top Microbiol Immunol. 2003. PMID: 12797445 Review.
-
Dendritic cells and measles virus infection.Curr Top Microbiol Immunol. 2003;276:77-101. doi: 10.1007/978-3-662-06508-2_4. Curr Top Microbiol Immunol. 2003. PMID: 12797444 Review.
-
Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression.Proc Natl Acad Sci U S A. 1997 May 13;94(10):5326-31. doi: 10.1073/pnas.94.10.5326. Proc Natl Acad Sci U S A. 1997. PMID: 9144236 Free PMC article.
-
Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells.J Immunol. 2000 Feb 15;164(4):1753-60. doi: 10.4049/jimmunol.164.4.1753. J Immunol. 2000. PMID: 10657621
-
Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus.J Virol. 2000 May;74(9):4387-93. doi: 10.1128/jvi.74.9.4387-4393.2000. J Virol. 2000. PMID: 10756053 Free PMC article.
Cited by
-
A prominent role for DC-SIGN+ dendritic cells in initiation and dissemination of measles virus infection in non-human primates.PLoS One. 2012;7(12):e49573. doi: 10.1371/journal.pone.0049573. Epub 2012 Dec 5. PLoS One. 2012. PMID: 23227146 Free PMC article.
-
Gene expression profiles identify inflammatory signatures in dendritic cells.PLoS One. 2010 Feb 24;5(2):e9404. doi: 10.1371/journal.pone.0009404. PLoS One. 2010. PMID: 20195376 Free PMC article.
-
Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus.PLoS Negl Trop Dis. 2007 Dec 12;1(3):e96. doi: 10.1371/journal.pntd.0000096. PLoS Negl Trop Dis. 2007. PMID: 18160988 Free PMC article.
-
Current perspectives in assessing humoral immunity after measles vaccination.Expert Rev Vaccines. 2019 Jan;18(1):75-87. doi: 10.1080/14760584.2019.1559063. Epub 2018 Dec 26. Expert Rev Vaccines. 2019. PMID: 30585753 Free PMC article. Review.
-
Development of human dendritic cells and their role in HIV infection: antiviral immunity versus HIV transmission.Front Microbiol. 2013 Jul 9;4:178. doi: 10.3389/fmicb.2013.00178. eCollection 2013. Front Microbiol. 2013. PMID: 23847602 Free PMC article.
References
-
- Stein C. E., Birmingham M., Kurian M., Duclos P., Strebel P. J. Infect. Dis. 2003;187(Suppl. 1):S8–S14. - PubMed
-
- Griffin D. E. Curr. Top. Microbiol. Immunol. 1995;191:117–134. - PubMed
-
- Esolen L. M., Ward B. J., Moench T. R., Griffin D. E. J. Infect. Dis. 1993;168:47–52. - PubMed
-
- Moench T. R., Griffin D. E., Obriecht C. R., Vaisberg A. J., Johnson R. T. J. Infect. Dis. 1988;158:433–442. - PubMed
-
- Schneider-Schaulies S., Bieback K., Avota E., Klagge I., ter Meulen V. J. Mol. Med. 2002;80:73–85. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

