Epstein-Barr virus provides a new paradigm: a requirement for the immediate inhibition of apoptosis
- PMID: 16277553
- PMCID: PMC1283332
- DOI: 10.1371/journal.pbio.0030404
Epstein-Barr virus provides a new paradigm: a requirement for the immediate inhibition of apoptosis
Abstract
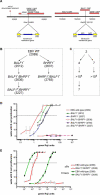
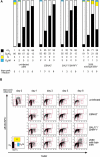
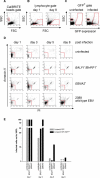
DNA viruses such as herpesviruses are known to encode homologs of cellular antiapoptotic viral Bcl-2 proteins (vBcl-2s), which protect the virus from apoptosis in its host cell during virus synthesis. Epstein-Barr virus (EBV), a human tumor virus and a prominent member of gamma-herpesviruses, infects primary resting B lymphocytes to establish a latent infection and yield proliferating, growth-transformed B cells in vitro. In these cells, 11 viral genes that contribute to cellular transformation are consistently expressed. EBV also encodes two vBcl-2 genes whose roles are unclear. Here we show that the genetic inactivation of both vBcl-2 genes disabled EBV's ability to transform primary resting B lymphocytes. Primary B cells infected with a vBcl-2-negative virus did not enter the cell cycle and died of immediate apoptosis. Apoptosis was abrogated in infected cells in which vBcl-2 genes were maximally expressed within the first 24 h postinfection. During latent infection, however, the expression of vBcl-2 genes became undetectable. Thus, both vBcl-2 homologs are essential for initial cellular transformation but become dispensable once a latent infection is established. Because long-lived, latently infected memory B cells and EBV-associated B-cell lymphomas are derived from EBV-infected proapoptotic germinal center B cells, we conclude that vBcl-2 genes are essential for the initial evasion of apoptosis in cells in vivo in which the virus establishes a latent infection or causes cellular transformation or both.
Figures

Similar articles
-
First Days in the Life of Naive Human B Lymphocytes Infected with Epstein-Barr Virus.mBio. 2019 Sep 17;10(5):e01723-19. doi: 10.1128/mBio.01723-19. mBio. 2019. PMID: 31530670 Free PMC article.
-
Human B cells on their route to latent infection--early but transient expression of lytic genes of Epstein-Barr virus.Eur J Cell Biol. 2012 Jan;91(1):65-9. doi: 10.1016/j.ejcb.2011.01.014. Epub 2011 Mar 29. Eur J Cell Biol. 2012. PMID: 21450364 Review.
-
Repression of the proapoptotic cellular BIK/NBK gene by Epstein-Barr virus antagonizes transforming growth factor β1-induced B-cell apoptosis.J Virol. 2014 May;88(9):5001-13. doi: 10.1128/JVI.03642-13. Epub 2014 Feb 19. J Virol. 2014. PMID: 24554662 Free PMC article.
-
Three restricted forms of Epstein-Barr virus latency counteracting apoptosis in c-myc-expressing Burkitt lymphoma cells.Proc Natl Acad Sci U S A. 2006 Oct 3;103(40):14935-40. doi: 10.1073/pnas.0509988103. Epub 2006 Sep 25. Proc Natl Acad Sci U S A. 2006. PMID: 17001014 Free PMC article.
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
Cited by
-
Molecular signature of Epstein Barr virus-positive Burkitt lymphoma and post-transplant lymphoproliferative disorder suggest different roles for Epstein Barr virus.Front Microbiol. 2014 Dec 23;5:728. doi: 10.3389/fmicb.2014.00728. eCollection 2014. Front Microbiol. 2014. PMID: 25566237 Free PMC article.
-
Shutoff of BZLF1 gene expression is necessary for immortalization of primary B cells by Epstein-Barr virus.J Virol. 2012 Aug;86(15):8086-96. doi: 10.1128/JVI.00234-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623769 Free PMC article.
-
Epstein-Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells.Nucleic Acids Res. 2010 Apr;38(6):1932-49. doi: 10.1093/nar/gkp1169. Epub 2009 Dec 23. Nucleic Acids Res. 2010. PMID: 20034954 Free PMC article.
-
Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68.PLoS Pathog. 2008 Feb 8;4(2):e25. doi: 10.1371/journal.ppat.0040025. PLoS Pathog. 2008. PMID: 18248095 Free PMC article.
-
Unexpected patterns of Epstein-Barr virus transcription revealed by a high throughput PCR array for absolute quantification of viral mRNA.Virology. 2015 Jan 1;474:117-30. doi: 10.1016/j.virol.2014.10.030. Epub 2014 Nov 15. Virology. 2015. PMID: 25463610 Free PMC article.
References
-
- Cuconati A, White E. Viral homologs of BCL-2: Role of apoptosis in the regulation of virus infection. Genes Dev. 2002;16:2465–2478. - PubMed
-
- Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, et al. Viral proteins targeting mitochondria: Controlling cell death. Biochim Biophys Acta. 2004;1659:178–189. - PubMed
-
- Benedict CA, Norris PS, Ware CF. To kill or be killed: Viral evasion of apoptosis. Nat Immunol. 2002;3:1013–1018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

