Ligation of CD8alpha on human natural killer cells prevents activation-induced apoptosis and enhances cytolytic activity
- PMID: 16236125
- PMCID: PMC1802415
- DOI: 10.1111/j.1365-2567.2005.02235.x
Ligation of CD8alpha on human natural killer cells prevents activation-induced apoptosis and enhances cytolytic activity
Abstract
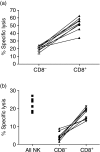
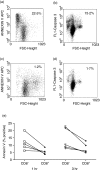
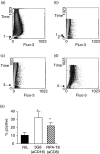
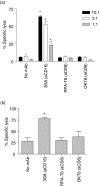
It has been previously shown that the subset of human natural killer (NK) cells which express CD8 in a homodimeric alpha/alpha form are more cytotoxic than their CD8- counterparts but the mechanisms behind this differential cytolytic activity remained unknown. Target cell lysis by CD8- NK cells is associated with high levels of effector cell apoptosis, which is in contrast to the significantly lower levels found in the CD8alpha+ cells after lysis of the same targets. We report that cross-linking of the CD8alpha chains on NK cells induces rapid rises in intracellular Ca2+ and increased expression of CD69 at the cell surface by initiating the influx of extracellular Ca2+ ions. We demonstrate that secretion of cytolytic enzymes initiates NK-cell apoptosis from which CD8alpha+ NK cells are protected by an influx of exogenous calcium following ligation of CD8 on the NK-cell surface. This ligation is through interaction with fellow NK cells in the cell conjugate and can occur when the target cells lack major histocompatibility complex (MHC) Class I expression. Protection from apoptosis is blocked by preincubation of the NK cells with anti-MHC Class I antibody. Thus, in contrast to the CD8- subset, CD8alpha+ NK cells are capable of sequential lysis of multiple target cells.
Figures
Similar articles
-
KHYG-1, a model for the study of enhanced natural killer cell cytotoxicity.Exp Hematol. 2005 Oct;33(10):1160-71. doi: 10.1016/j.exphem.2005.06.024. Exp Hematol. 2005. PMID: 16219538
-
The binding and lysis of target cells by cytotoxic lymphocytes: molecular and cellular aspects.Annu Rev Immunol. 1994;12:735-73. doi: 10.1146/annurev.iy.12.040194.003511. Annu Rev Immunol. 1994. PMID: 8011296 Review.
-
Human CD56bright and CD56dim natural killer cell subsets respond differentially to direct stimulation with Mycobacterium bovis bacillus Calmette-Guérin.Scand J Immunol. 2005 Dec;62(6):498-506. doi: 10.1111/j.1365-3083.2005.01692.x. Scand J Immunol. 2005. PMID: 16316416
-
Cellular mechanisms of lymphocyte-mediated lysis of tumor cells.Ann Ist Super Sanita. 1990;26(3-4):369-84. Ann Ist Super Sanita. 1990. PMID: 2151107 Review.
-
Soluble HLA-I-mediated secretion of TGF-beta1 by human NK cells and consequent down-regulation of anti-tumor cytolytic activity.Eur J Immunol. 2009 Dec;39(12):3459-68. doi: 10.1002/eji.200939728. Eur J Immunol. 2009. PMID: 19830740
Cited by
-
Loss of circulating CD8α+ NK cells during human Mycobacterium tuberculosis infection.bioRxiv [Preprint]. 2024 May 13:2024.04.16.588542. doi: 10.1101/2024.04.16.588542. bioRxiv. 2024. PMID: 38659858 Free PMC article. Preprint.
-
Effect of Granzyme K, FasL and Interferon-γ Expression in Placentas with Preeclampsia.Biomedicines. 2024 Apr 11;12(4):842. doi: 10.3390/biomedicines12040842. Biomedicines. 2024. PMID: 38672196 Free PMC article.
-
Adapting the SMART tube technology for flow cytometry in feline full blood samples.Front Vet Sci. 2024 Jun 26;11:1377414. doi: 10.3389/fvets.2024.1377414. eCollection 2024. Front Vet Sci. 2024. PMID: 38988976 Free PMC article.
-
High-dimensional mass cytometry reveals systemic and local immune signatures in necrotizing enterocolitis.Front Immunol. 2023 Nov 17;14:1292987. doi: 10.3389/fimmu.2023.1292987. eCollection 2023. Front Immunol. 2023. PMID: 38045686 Free PMC article.
-
Multidimensional Analyses of Donor Memory-Like NK Cells Reveal New Associations with Response after Adoptive Immunotherapy for Leukemia.Cancer Discov. 2020 Dec;10(12):1854-1871. doi: 10.1158/2159-8290.CD-20-0312. Epub 2020 Aug 21. Cancer Discov. 2020. PMID: 32826231 Free PMC article.
References
-
- Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–8. - PubMed
-
- Miceli MC, Parnes JR. Role of CD4 and CD8 in T cell activation and differentiation. Adv Immunol. 1993;53:59–122. - PubMed
-
- Parnes JR. Molecular biology and function of CD4 and CD8. Adv Immunol. 1989;44:265–311. - PubMed
-
- Kushima K, Fujita M, Shigeta A, Horiuchi H, Matsuda H, Furusawa S. Flow cytometric analysis of chicken NK activity and its use on the effect of restraint stress. J Vet Med Sci. 2003;65:995–1000. - PubMed
-
- Srour EF, Leemhuis T, Jenski L, Redmond R, Jansen J. Cytolytic activity of human natural killer cells subpopulations by four-colour immunofluorescence flow cytometric sorting. Cytometry. 1990;11:442–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

