The mechanism of pneumolysin-induced cochlear hair cell death in the rat
- PMID: 16051626
- PMCID: PMC1474774
- DOI: 10.1113/jphysiol.2005.092478
The mechanism of pneumolysin-induced cochlear hair cell death in the rat
Abstract
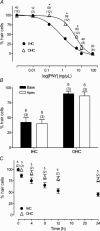
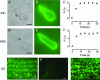
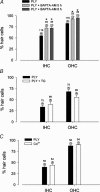
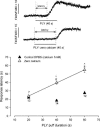
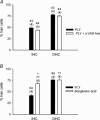
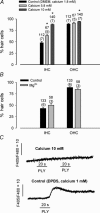
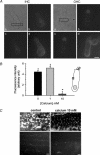
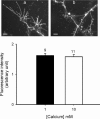
Streptoccocus pneumoniae infection can result in local and systemic diseases such as otitis media, pneumonia and meningitis. Sensorineural hearing loss associated with this infection is mediated by the release of an exotoxin, pneumolysin. The goal of the present study was to characterize the mechanisms of pneumolysin toxicity in cochlear hair cells in vitro. Pneumolysin induced severe damage in cochlear hair cells, ranging from stereocilia disorganization to total cell loss. Surprisingly, pneumolysin-induced cell death preferentially targeted inner hair cells. Pneumolysin triggered in vitro cell death by an influx of calcium. Extracellular calcium appeared to enter the cell through a pore formed by the toxin. Buffering intracellular calcium with BAPTA improved hair cell survival. The mitochondrial apoptotic pathway involved in pneumolysin-induced cell death was demonstrated by the use of bongkrekic acid. Binding of pneumolysin to the hair cell plasma membrane was required to induce cell death. Increasing external calcium reduced cell toxicity by preventing the binding of pneumolysin to hair cell membranes. These results showed the significant role of calcium both in triggering pneumolysin-induced hair cell apoptosis and in preventing the toxin from binding to its cellular target.
Figures


Similar articles
-
Zinc protection against pneumolysin toxicity on rat cochlear hair cells.Audiol Neurootol. 2008;13(1):65-70. doi: 10.1159/000108763. Epub 2007 Sep 21. Audiol Neurootol. 2008. PMID: 17890859
-
Intracochlear perfusion of pneumolysin, a pneumococcal protein, rapidly abolishes auditory potentials in the Guinea pig cochlea.Acta Otolaryngol. 2004 Nov;124(9):1000-7. doi: 10.1080/00016480410017125. Acta Otolaryngol. 2004. PMID: 15513541
-
Development and properties of stereociliary link types in hair cells of the mouse cochlea.J Comp Neurol. 2005 Apr 25;485(1):75-85. doi: 10.1002/cne.20513. J Comp Neurol. 2005. PMID: 15776440
-
Tonotopy in calcium homeostasis and vulnerability of cochlear hair cells.Hear Res. 2019 May;376:11-21. doi: 10.1016/j.heares.2018.11.002. Epub 2018 Nov 16. Hear Res. 2019. PMID: 30473131 Free PMC article. Review.
-
The significance of the calcium signal in the outer hair cells and its possible role in tinnitus of cochlear origin.Eur Arch Otorhinolaryngol. 2004 Nov;261(10):517-25. doi: 10.1007/s00405-004-0745-9. Epub 2004 Sep 29. Eur Arch Otorhinolaryngol. 2004. PMID: 15609110 Review.
Cited by
-
Multi-Valent Protein Hybrid Pneumococcal Vaccines: A Strategy for the Next Generation of Vaccines.Vaccines (Basel). 2021 Mar 2;9(3):209. doi: 10.3390/vaccines9030209. Vaccines (Basel). 2021. PMID: 33801372 Free PMC article. Review.
-
Pneumolysin: Pathogenesis and Therapeutic Target.Front Microbiol. 2020 Jul 2;11:1543. doi: 10.3389/fmicb.2020.01543. eCollection 2020. Front Microbiol. 2020. PMID: 32714314 Free PMC article. Review.
-
Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection.PLoS Pathog. 2015 May 14;11(5):e1004836. doi: 10.1371/journal.ppat.1004836. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25973949 Free PMC article.
-
Pneumococcal Surface Proteins as Virulence Factors, Immunogens, and Conserved Vaccine Targets.Front Cell Infect Microbiol. 2022 May 12;12:832254. doi: 10.3389/fcimb.2022.832254. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35646747 Free PMC article. Review.
-
Pneumolysin activates macrophage lysosomal membrane permeabilization and executes apoptosis by distinct mechanisms without membrane pore formation.mBio. 2014 Oct 7;5(5):e01710-14. doi: 10.1128/mBio.01710-14. mBio. 2014. PMID: 25293758 Free PMC article.
References
-
- Alouf J. Cholesterol-binding cytolytic protein toxins. Int J Med Microbiol. 2000;290:351–356. - PubMed
-
- Andrew PW, Mitchell TJ, Morgan PJ. Relationship of structure to function in pneumolysin. Microb Drug Resist. 1997;3:11–17. - PubMed
-
- Bhatt S, Halpin C, Hsu W, Thedinger BA, Levine RA, Tuomanen E, Nadol JB. Hearing loss and pneumococcal meningitis: an animal model. Laryngoscope. 1991;101:1285–1292. - PubMed
-
- Bhatt SM, Lauretano A, Cabellos C, Halpin C, Levine RA, Xu WZ, Nadol JB, Tuomanen E. Progression of hearing loss in experimental pneumococcal meningitis: correlation with cerebrospinal fluid cytochemistry. J Infect Dis. 1993;167:675–683. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

