Distinct mechanisms of CD4+ and CD8+ T-cell activation and bystander apoptosis induced by human immunodeficiency virus type 1 virions
- PMID: 15858014
- PMCID: PMC1091688
- DOI: 10.1128/JVI.79.10.6299-6311.2005
Distinct mechanisms of CD4+ and CD8+ T-cell activation and bystander apoptosis induced by human immunodeficiency virus type 1 virions
Abstract
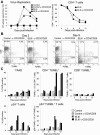
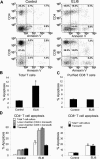
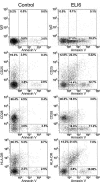
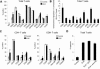
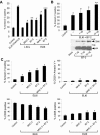
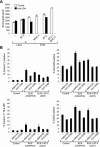
Apoptosis of uninfected bystander T cells contributes to T-cell depletion during human immunodeficiency virus type 1 (HIV-1) infection. HIV-1 envelope/receptor interactions and immune activation have been implicated as contributors to bystander apoptosis. To better understand the relationship between T-cell activation and bystander apoptosis during HIV-1 pathogenesis, we investigated the effects of the highly cytopathic CXCR4-tropic HIV-1 variant ELI6 on primary CD4(+) and CD8(+) T cells. Infection of primary T-cell cultures with ELI6 induced CD4(+) T-cell depletion by direct cell lysis and bystander apoptosis. Exposure of primary CD4(+) and CD8(+) T cells to nonreplicating ELI6 virions induced bystander apoptosis through a Fas-independent mechanism. Bystander apoptosis of CD4(+) T cells required direct contact with virions and Env/CXCR4 binding. In contrast, the apoptosis of CD8(+) T cells was triggered by a soluble factor(s) secreted by CD4(+) T cells. HIV-1 virions activated CD4(+) and CD8(+) T cells to express CD25 and HLA-DR and preferentially induced apoptosis in CD25(+)HLA-DR(+) T cells in a CXCR4-dependent manner. Maximal levels of binding, activation, and apoptosis were induced by virions that incorporated MHC class II and B7-2 into the viral membrane. These results suggest that nonreplicating HIV-1 virions contribute to chronic immune activation and T-cell depletion during HIV-1 pathogenesis by activating CD4(+) and CD8(+) T cells, which then proceed to die via apoptosis. This mechanism may represent a viral immune evasion strategy to increase viral replication by activating target cells while killing immune effector cells that are not productively infected.
Figures

Similar articles
-
Partial activation and induction of apoptosis in CD4(+) and CD8(+) T lymphocytes by conformationally authentic noninfectious human immunodeficiency virus type 1.J Virol. 2001 Feb;75(3):1152-64. doi: 10.1128/JVI.75.3.1152-1164.2001. J Virol. 2001. PMID: 11152488 Free PMC article.
-
Apoptosis of bystander T cells induced by human immunodeficiency virus type 1 with increased envelope/receptor affinity and coreceptor binding site exposure.J Virol. 2004 May;78(9):4541-51. doi: 10.1128/jvi.78.9.4541-4551.2004. J Virol. 2004. PMID: 15078935 Free PMC article.
-
Apoptotic killing of CD4+ T lymphocytes in HIV-1-infected PHA-stimulated PBL cultures is mediated by CD8+ LAK cells.Virology. 1998 Feb 15;241(2):169-80. doi: 10.1006/viro.1997.8979. Virology. 1998. PMID: 9499792
-
Host and Viral Factors in HIV-Mediated Bystander Apoptosis.Viruses. 2017 Aug 22;9(8):237. doi: 10.3390/v9080237. Viruses. 2017. PMID: 28829402 Free PMC article. Review.
-
CD8 lymphocytes in HIV infection: helpful and harmful.J Clin Lab Immunol. 1997;49(1):15-32. J Clin Lab Immunol. 1997. PMID: 9819670 Review.
Cited by
-
Role of apoptosis in disease.Aging (Albany NY). 2012 May;4(5):330-49. doi: 10.18632/aging.100459. Aging (Albany NY). 2012. PMID: 22683550 Free PMC article. Review.
-
Casp8p41: The Protean Mediator of Death in CD4 T-cells that Replicate HIV.J Cell Death. 2016 Sep 27;9:9-17. doi: 10.4137/JCD.S39872. eCollection 2016. J Cell Death. 2016. PMID: 27721655 Free PMC article. Review.
-
Increased metallothionein gene expression, zinc, and zinc-dependent resistance to apoptosis in circulating monocytes during HIV viremia.J Leukoc Biol. 2010 Sep;88(3):589-96. doi: 10.1189/jlb.0110051. Epub 2010 Jun 15. J Leukoc Biol. 2010. PMID: 20551211 Free PMC article.
-
Distinctive in vitro effects of T-cell growth cytokines on cytomegalovirus-stimulated T-cell responses of HIV-infected HAART recipients.Virology. 2008 Aug 15;378(1):48-57. doi: 10.1016/j.virol.2008.05.018. Epub 2008 Jun 24. Virology. 2008. PMID: 18572217 Free PMC article.
-
A machine learning approach for identifying amino acid signatures in the HIV env gene predictive of dementia.PLoS One. 2012;7(11):e49538. doi: 10.1371/journal.pone.0049538. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23166702 Free PMC article.
References
-
- Algeciras-Schimnich, A., S. R. Vlahakis, A. Villasis-Keever, T. Gomez, C. J. Heppelmann, G. Bou, and C. V. Paya. 2002. CCR5-mediates Fas- and caspase-8 dependent apoptosis of both uninfected and HIV infected primary human CD4 T cells. AIDS 16:1467-1478. - PubMed
-
- Arthur, L. O., J. W. Bess, Jr., R. C. Sowder II, R. E. Benveniste, L. E. Henderson, and J. D. Lifson. 1992. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science 258:1935-1938. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

