RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model
- PMID: 15811941
- PMCID: PMC556303
- DOI: 10.1073/pnas.0501507102
RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model
Abstract
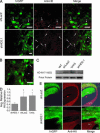
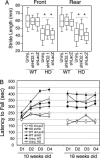
Huntington's disease (HD) is a fatal, dominant neurogenetic disorder. HD results from polyglutamine repeat expansion (CAG codon, Q) in exon 1 of HD, conferring a toxic gain of function on the protein huntingtin (htt). Currently, no preventative treatment exists for HD. RNA interference (RNAi) has emerged as a potential therapeutic tool for treating dominant diseases by directly reducing disease gene expression. Here, we show that RNAi directed against mutant human htt reduced htt mRNA and protein expression in cell culture and in HD mouse brain. Importantly, htt gene silencing improved behavioral and neuropathological abnormalities associated with HD. Our data provide support for the further development of RNAi for HD therapy.
Figures

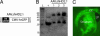
Similar articles
-
Sustained effects of nonallele-specific Huntingtin silencing.Ann Neurol. 2009 Mar;65(3):276-85. doi: 10.1002/ana.21569. Ann Neurol. 2009. PMID: 19334076
-
Intrajugular vein delivery of AAV9-RNAi prevents neuropathological changes and weight loss in Huntington's disease mice.Mol Ther. 2014 Apr;22(4):797-810. doi: 10.1038/mt.2013.289. Epub 2014 Jan 6. Mol Ther. 2014. PMID: 24390280 Free PMC article.
-
Preclinical safety of RNAi-mediated HTT suppression in the rhesus macaque as a potential therapy for Huntington's disease.Mol Ther. 2011 Dec;19(12):2152-62. doi: 10.1038/mt.2011.219. Epub 2011 Oct 25. Mol Ther. 2011. PMID: 22031240 Free PMC article.
-
Delivering a disease-modifying treatment for Huntington's disease.Drug Discov Today. 2015 Jan;20(1):50-64. doi: 10.1016/j.drudis.2014.09.011. Epub 2014 Sep 26. Drug Discov Today. 2015. PMID: 25256777 Review.
-
RNAi-based therapies for Huntington's disease: delivery challenges and opportunities.Ther Deliv. 2012 Sep;3(9):1061-76. doi: 10.4155/tde.12.80. Ther Deliv. 2012. PMID: 23035592 Review.
Cited by
-
Sustained therapeutic reversal of Huntington's disease by transient repression of huntingtin synthesis.Neuron. 2012 Jun 21;74(6):1031-44. doi: 10.1016/j.neuron.2012.05.009. Neuron. 2012. PMID: 22726834 Free PMC article.
-
Stem Cell-Derived Exosomes: a New Strategy of Neurodegenerative Disease Treatment.Mol Neurobiol. 2021 Jul;58(7):3494-3514. doi: 10.1007/s12035-021-02324-x. Epub 2021 Mar 21. Mol Neurobiol. 2021. PMID: 33745116 Free PMC article. Review.
-
Therapeutic targeting of RNA for neurological and neuromuscular disease.Genes Dev. 2024 Sep 19;38(15-16):698-717. doi: 10.1101/gad.351612.124. Genes Dev. 2024. PMID: 39142832 Free PMC article. Review.
-
Protein misfolding detected early in pathogenesis of transgenic mouse model of Huntington disease using amyloid seeding assay.J Biol Chem. 2012 Mar 23;287(13):9982-9989. doi: 10.1074/jbc.M111.305417. Epub 2011 Dec 20. J Biol Chem. 2012. PMID: 22187438 Free PMC article.
-
Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing.Mol Ther. 2016 Oct;24(10):1836-1847. doi: 10.1038/mt.2016.126. Epub 2016 Jun 27. Mol Ther. 2016. PMID: 27506293 Free PMC article.
References
-
- The Huntington's Disease Collaborative Research Group (1993) Cell 72, 971–983. - PubMed
-
- Gusella, J. F. & MacDonald, M. E. (2000) Nat. Rev. Neurosci. 1, 109–115. - PubMed
-
- Ona, V. O., Li, M., Vonsattel, J. P., Andrews, L. J., Khan, S. Q., Chung, W. M., Frey, A. S., Menon, A. S., Li, X. J., Stieg, P. E., et al. (1999) Nature 399, 263–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

