Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization
- PMID: 15753206
- PMCID: PMC2212841
- DOI: 10.1084/jem.20041982
Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization
Abstract
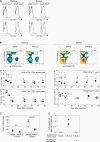
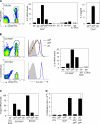
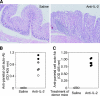
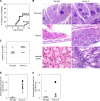
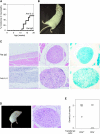
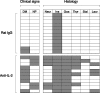
Interleukin (IL)-2 plays a crucial role in the maintenance of natural immunologic self-tolerance. Neutralization of circulating IL-2 by anti-IL-2 monoclonal antibody for a limited period elicits autoimmune gastritis in BALB/c mice. Similar treatment of diabetes-prone nonobese diabetic mice triggers early onset of diabetes and produces a wide spectrum of T cell-mediated autoimmune diseases, including gastritis, thyroiditis, sialadenitis, and notably, severe neuropathy. Such treatment selectively reduces the number of Foxp3-expressing CD25(+) CD4(+) T cells, but not CD25(-) CD4(+) T cells, in the thymus and periphery of normal and thymectomized mice. IL-2 neutralization inhibits physiological proliferation of peripheral CD25(+) CD4(+) T cells that are presumably responding to normal self-antigens, whereas it is unable to inhibit their lymphopenia-induced homeostatic expansion in a T cell-deficient environment. In normal naive mice, CD25(low) CD4(+) nonregulatory T cells actively transcribe the IL-2 gene and secrete IL-2 protein in the physiological state. IL-2 is thus indispensable for the peripheral maintenance of natural CD25(+) CD4(+) regulatory T cells (T reg cells). The principal physiological source of IL-2 for the maintenance of T reg cells appears to be other T cells, especially CD25(low) CD4(+) activated T cells, which include self-reactive T cells. Furthermore, impairment of this negative feedback loop via IL-2 can be a cause and a predisposing factor for autoimmune disease.
Figures

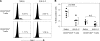
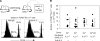
Similar articles
-
Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells.Nat Med. 2005 Nov;11(11):1238-43. doi: 10.1038/nm1312. Epub 2005 Oct 16. Nat Med. 2005. PMID: 16227988
-
The role of CD4+CD25+ immunoregulatory T cells in the induction of autoimmune gastritis.Immunol Cell Biol. 2002 Dec;80(6):567-73. doi: 10.1046/j.1440-1711.2002.01127.x. Immunol Cell Biol. 2002. PMID: 12406391
-
Foxp3+ CD25- CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion.Proc Natl Acad Sci U S A. 2005 Mar 15;102(11):4091-6. doi: 10.1073/pnas.0408679102. Epub 2005 Mar 7. Proc Natl Acad Sci U S A. 2005. PMID: 15753306 Free PMC article.
-
Natural regulatory CD4 T cells expressing CD25.Microbes Infect. 2001 Sep;3(11):937-45. doi: 10.1016/s1286-4579(01)01455-1. Microbes Infect. 2001. PMID: 11564442 Review.
-
Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease.Immunol Rev. 2006 Aug;212:8-27. doi: 10.1111/j.0105-2896.2006.00427.x. Immunol Rev. 2006. PMID: 16903903 Review.
Cited by
-
The role of cytokine signaling in the pathogenesis of cutaneous T-cell lymphoma.Cancer Biol Ther. 2011 Dec 15;12(12):1019-22. doi: 10.4161/cbt.12.12.18144. Epub 2011 Dec 15. Cancer Biol Ther. 2011. PMID: 22236880 Free PMC article. Review.
-
Adoptive T Regulatory Cell Therapy for Tolerance Induction.Curr Transplant Rep. 2015 Jun 1;2(2):191-201. doi: 10.1007/s40472-015-0058-5. Curr Transplant Rep. 2015. PMID: 25938011 Free PMC article.
-
Regulatory T cells turn pathogenic.Cell Mol Immunol. 2015 Sep;12(5):525-32. doi: 10.1038/cmi.2015.12. Epub 2015 Mar 16. Cell Mol Immunol. 2015. PMID: 25942597 Free PMC article. Review.
-
TNFSF9 Is Associated with Favorable Tumor Immune Microenvironment in Patients with Renal Cell Carcinoma Who Are Treated with the Combination Therapy of Nivolumab and Ipilimumab.Int J Mol Sci. 2024 Jul 6;25(13):7444. doi: 10.3390/ijms25137444. Int J Mol Sci. 2024. PMID: 39000552 Free PMC article.
-
From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines.Nat Immunol. 2012 Oct;13(10):925-31. doi: 10.1038/ni.2406. Epub 2012 Sep 18. Nat Immunol. 2012. PMID: 22990890 Free PMC article. Review.
References
-
- Horak, I., J. Lohler, A. Ma, and K.A. Smith. 1995. Interleukin-2 deficient mice: a new model to study autoimmunity and self-tolerance. Immunol. Rev. 148:35–44. - PubMed
-
- Serreze, D.V., K. Hamaguchi, and E.H. Leiter. 1989. Immunostimulation circumvents diabetes in NOD/Lt mice. J. Autoimmun. 2:759–776. - PubMed
-
- Gutierrez-Ramos, J.C., J.L. Andreu, Y. Revilla, E. Vinuela, and C. Martinez. 1990. Recovery from autoimmunity of MRL/lpr mice after infection with an interleukin-2/vaccinia recombinant virus. Nature. 346:271–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

