R5 human immunodeficiency virus type 1 infection of fetal thymic organ culture induces cytokine and CCR5 expression
- PMID: 15596839
- PMCID: PMC538709
- DOI: 10.1128/JVI.79.1.458-471.2005
R5 human immunodeficiency virus type 1 infection of fetal thymic organ culture induces cytokine and CCR5 expression
Abstract
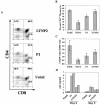
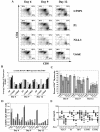
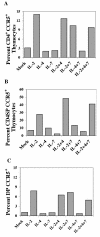
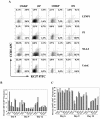
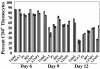
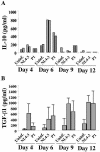
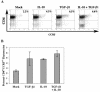
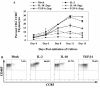
Late-stage CCR5 tropic human immunodeficiency virus type 1 (HIV-1) isolates (R5 HIV-1) can deplete nearly all CD4+ thymocytes from human thymus/liver grafts, despite the fact that fewer than 5% of these cells express CCR5. To resolve this paradox, we studied the replication and cytopathic effects (CPE) of late-stage R5 HIV-1 biological clones from two progressors and two long-term nonprogressors (LTNP) in fetal thymic organ culture (FTOC) with and without added cytokines. We found that R5 HIV-1 clones from progressors but not LTNP were cytopathic in untreated FTOC. Moreover, R5 HIV-1 clones from progressors replicated to higher levels than LTNP-derived R5 HIV-1 clones in this system. In contrast, when FTOC was maintained in the presence of interleukin 2 (IL-2), IL-4, and IL-7, both progressor and LTNP clones exhibited similar replication and CPE, which were equal to or greater than the levels achieved by progressor-derived R5 HIV-1 clones in untreated FTOC. This finding was likely due to IL-2-induced CCR5 expression on CD4+ thymocytes in FTOC. R5 HIV-1 clones showed greater pathogenesis for CCR5+ cells but also showed evidence of CPE on CCR5- cells. Furthermore, infection of FTOC by R5 HIV-1 induced IL-10 and transforming growth factor beta (TGF-beta) expression. Both IL-10 and TGF-beta in turn induced CCR5 expression in FTOC. Induction of CCR5 expression via cytokine induction by R5 HIV-1 infection of CCR5+ thymocytes likely permitted further viral replication in newly CCR5+ thymocytes. CCR5 expression, therefore, is a key determinant of pathogenesis of R5 HIV-1 in FTOC.
Figures
Similar articles
-
The envelope gene is a cytopathic determinant of CCR5 tropic HIV-1.Virology. 2007 Feb 5;358(1):23-38. doi: 10.1016/j.virol.2006.08.027. Epub 2006 Sep 26. Virology. 2007. PMID: 16999983
-
Pathogenesis of primary R5 human immunodeficiency virus type 1 clones in SCID-hu mice.J Virol. 2000 Apr;74(7):3205-16. doi: 10.1128/jvi.74.7.3205-3216.2000. J Virol. 2000. PMID: 10708437 Free PMC article.
-
Impact of cytokines on replication in the thymus of primary human immunodeficiency virus type 1 isolates from infants.J Virol. 2002 Jul;76(14):6929-43. doi: 10.1128/jvi.76.14.6929-6943.2002. J Virol. 2002. PMID: 12072494 Free PMC article.
-
New players in cytokine control of HIV infection.Curr HIV/AIDS Rep. 2008 Feb;5(1):27-32. doi: 10.1007/s11904-008-0005-5. Curr HIV/AIDS Rep. 2008. PMID: 18417032 Review.
-
HIV-1 replication and pathogenesis in the human thymus.Curr HIV Res. 2003 Jul;1(3):275-85. doi: 10.2174/1570162033485258. Curr HIV Res. 2003. PMID: 15046252 Free PMC article. Review.
Cited by
-
Low immune activation despite high levels of pathogenic human immunodeficiency virus type 1 results in long-term asymptomatic disease.J Virol. 2007 Aug;81(16):8838-42. doi: 10.1128/JVI.02663-06. Epub 2007 May 30. J Virol. 2007. PMID: 17537849 Free PMC article.
-
Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo.Nat Biotechnol. 2010 Aug;28(8):839-47. doi: 10.1038/nbt.1663. Epub 2010 Jul 2. Nat Biotechnol. 2010. PMID: 20601939 Free PMC article.
-
Comparison of the Biological Basis for Non-HIV Transmission to HIV-Exposed Seronegative Individuals, Disease Non-Progression in HIV Long-Term Non-Progressors and Elite Controllers.Viruses. 2023 Jun 13;15(6):1362. doi: 10.3390/v15061362. Viruses. 2023. PMID: 37376660 Free PMC article. Review.
-
Host and Viral Factors in HIV-Mediated Bystander Apoptosis.Viruses. 2017 Aug 22;9(8):237. doi: 10.3390/v9080237. Viruses. 2017. PMID: 28829402 Free PMC article. Review.
-
Differential pathogenesis of primary CCR5-using human immunodeficiency virus type 1 isolates in ex vivo human lymphoid tissue.J Virol. 2005 Sep;79(17):11151-60. doi: 10.1128/JVI.79.17.11151-11160.2005. J Virol. 2005. PMID: 16103166 Free PMC article.
References
-
- Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. - PubMed
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
-
- Berkowitz, R. D., K. P. Beckerman, T. J. Schall, and J. M. McCune. 1998. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J. Immunol. 161:3702-3710. - PubMed
-
- Blaak, H., M. Brouwer, L. J. Ran, F. de Wolf, and H. Schuitemaker. 1998. In vitro replication kinetics of human immunodeficiency virus type 1 (HIV-1) variants in relation to virus load in long-term survivors of HIV-1 infection. J. Infect. Dis. 177:600-610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

