A DNAbeta associated with Tomato yellow leaf curl China virus is required for symptom induction
- PMID: 15564504
- PMCID: PMC533896
- DOI: 10.1128/JVI.78.24.13966-13974.2004
A DNAbeta associated with Tomato yellow leaf curl China virus is required for symptom induction
Abstract
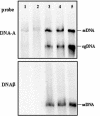
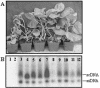
We report here that all 25 isolates of Tomato yellow leaf curl China virus (TYLCCNV) collected from tobacco, tomato, or Siegesbeckia orientalis plants in different regions of Yunnan Province, China, were associated with DNAbeta molecules. To investigate the biological role of DNAbeta, full-length infectious clones of viral DNA and DNAbeta of TYLCCNV isolate Y10 (TYLCCNV-Y10) were agroinoculated into Nicotiana benthamiana, Nicotiana glutinosa, Nicotiana. tabacum Samsun (NN or nn), tomato, and petunia plants. We found that TYLCCNV-Y10 alone could systemically infect these plants, but no symptoms were induced. TYLCCNV-Y10 DNAbeta was required, in addition to TYLCCNV-Y10, for induction of leaf curl disease in these hosts. Similar to TYLCCNV-Y10, DNAbeta of TYLCCNV isolate Y64 was also found to be required for induction of typical leaf curl diseases in the hosts tested. When the betaC1 gene of TYLCCNV-Y10 DNAbeta was mutated, the mutants failed to induce leaf curl symptoms in N. benthamiana when coinoculated with TYLCCNV-Y10. However, Southern blot hybridization analyses showed that the mutated DNAbeta molecules were replicated. When N. benthamiana and N. tabacum plants were transformed with a construct containing the betaC1 gene under the control of the Cauliflower mosaic virus 35S promoter, many transgenic plants developed leaf curl symptoms similar to those caused by a virus, the severity of which paralleled the level of betaC1 transcripts, while transgenic plants transformed with the betaC1 gene containing a stop codon after the start codon remained symptomless. Thus, expression of a betaC1 gene is adequate for induction of symptoms of viral infection in the absence of virus.
Figures




Similar articles
-
Pathogenicity and stability of a truncated DNAbeta associated with Tomato yellow leaf curl China virus.Virus Res. 2005 May;109(2):159-63. doi: 10.1016/j.virusres.2004.11.017. Epub 2005 Jan 8. Virus Res. 2005. PMID: 15763146
-
A Begomovirus DNAbeta-encoded protein binds DNA, functions as a suppressor of RNA silencing, and targets the cell nucleus.J Virol. 2005 Aug;79(16):10764-75. doi: 10.1128/JVI.79.16.10764-10775.2005. J Virol. 2005. PMID: 16051868 Free PMC article.
-
A modified viral satellite DNA that suppresses gene expression in plants.Plant J. 2004 Jun;38(5):850-60. doi: 10.1111/j.1365-313X.2004.02087.x. Plant J. 2004. PMID: 15144385
-
Expression of a begomoviral DNAbeta gene in transgenic Nicotiana plants induced abnormal cell division.J Zhejiang Univ Sci B. 2005 Feb;6(2):83-6. doi: 10.1631/jzus.2005.B0083. J Zhejiang Univ Sci B. 2005. PMID: 15633241 Free PMC article.
-
Trans-replication of, and competition between, DNA beta satellites in plants inoculated with Tomato yellow leaf curl China virus and Tobacco curly shoot virus.Phytopathology. 2009 Jun;99(6):716-20. doi: 10.1094/PHYTO-99-6-0716. Phytopathology. 2009. PMID: 19453231
Cited by
-
Recovery of Nicotiana benthamiana plants from a necrotic response induced by a nepovirus is associated with RNA silencing but not with reduced virus titer.J Virol. 2007 Nov;81(22):12285-97. doi: 10.1128/JVI.01192-07. Epub 2007 Aug 29. J Virol. 2007. PMID: 17728227 Free PMC article.
-
Viruses mobilize plant immunity to deter nonvector insect herbivores.Sci Adv. 2019 Aug 21;5(8):eaav9801. doi: 10.1126/sciadv.aav9801. eCollection 2019 Aug. Sci Adv. 2019. PMID: 31457079 Free PMC article.
-
Agroinfection of sweet potato by vacuum infiltration of an infectious sweepovirus.Virol Sin. 2014 Jun;29(3):148-54. doi: 10.1007/s12250-014-3430-1. Epub 2014 May 14. Virol Sin. 2014. PMID: 24903591 Free PMC article.
-
Vector development and vitellogenin determine the transovarial transmission of begomoviruses.Proc Natl Acad Sci U S A. 2017 Jun 27;114(26):6746-6751. doi: 10.1073/pnas.1701720114. Epub 2017 Jun 12. Proc Natl Acad Sci U S A. 2017. PMID: 28607073 Free PMC article.
-
Red-light is an environmental effector for mutualism between begomovirus and its vector whitefly.PLoS Pathog. 2021 Jan 11;17(1):e1008770. doi: 10.1371/journal.ppat.1008770. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33428670 Free PMC article.
References
-
- Arguello-Astorga, G. R., and R. Ruiz-Medrano. 2001. An iteron-related domain is associated to Motif 1 in the replication proteins of geminiviruses: identification of potential interacting amino acid-base pairs by a comparative approach. Arch. Virol. 146:1465-1485. - PubMed
-
- Briddon, R., S. Bull, I. Amin, A. Idris, S. Mansoor, I. Bedford, P. Dhawan, N. Rishi, S. Siwatch, A. Abdel-Salam, J. Brown, Y. Zafar, and P. Markham. 2003. Diversity of DNAβ, a satellite molecule associated with some monopartite begomoviruses. Virology 312:106-121. - PubMed
-
- Briddon, R. W., S. Mansoor, I. D. Bedford, M. S. Pinner, K. Saunders, J. Stanley, Y. Zafar, K. A. Malik, and P. G. Markham. 2001. Identification of DNA components required for induction of cotton leaf curl disease. Virology 285:234-243. - PubMed
-
- Duan, Y. P., C. A. Powell, D. E. Purcifull, P. Broglio, and E. Hiebert. 1997. Phenotypic variation in transgenic tobacco expressing mutated geminivirus movement/pathogenicity (BC1) proteins. Mol. Plant-Microbe Interact. 10:1065-1074. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

