Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation
- PMID: 15380353
- PMCID: PMC3147029
- DOI: 10.1016/j.virol.2004.07.008
Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation
Abstract
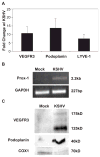
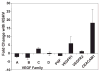
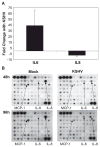
Kaposi's sarcoma-associated herpesvirus (KSHV) is necessary for KS, a highly vascularized tumor predominated by endothelial-derived spindle cells that express markers of lymphatic endothelium. Following KSHV infection of TIME cells, an immortalized human dermal microvascular endothelial cell (DMVEC) line, expression of many genes specific to lymphatic endothelium, including VEGFR3, podoplanin, LYVE-1, and Prox-1, is significantly increased. Increases in VEGFR3 and podoplanin protein are also demonstrated following latent infection. Examination of cytokine secretion showed that KSHV infection significantly induces hIL-6 while strongly inhibiting secretion of IL-8, a gene product that is decreased by differentiation of blood to lymphatic endothelial cells. These studies support the hypotheses that latent KSHV infection of blood endothelial cells drives their differentiation to lymphatic endothelial cells.
Copyright 2004 Elsevier Inc.
Figures

Similar articles
-
Ets-1 is required for the activation of VEGFR3 during latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells.J Virol. 2013 Jun;87(12):6758-68. doi: 10.1128/JVI.03241-12. Epub 2013 Apr 3. J Virol. 2013. PMID: 23552426 Free PMC article.
-
Kaposi's sarcoma herpesvirus microRNAs induce metabolic transformation of infected cells.PLoS Pathog. 2014 Sep 25;10(9):e1004400. doi: 10.1371/journal.ppat.1004400. eCollection 2014 Sep. PLoS Pathog. 2014. PMID: 25255370 Free PMC article.
-
Activation of Akt through gp130 receptor signaling is required for Kaposi's sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells.J Virol. 2008 Sep;82(17):8771-9. doi: 10.1128/JVI.00766-08. Epub 2008 Jun 25. J Virol. 2008. PMID: 18579585 Free PMC article.
-
Manipulation of endothelial cells by KSHV: implications for angiogenesis and aberrant vascular differentiation.Semin Cancer Biol. 2014 Jun;26:69-77. doi: 10.1016/j.semcancer.2014.01.008. Epub 2014 Jan 28. Semin Cancer Biol. 2014. PMID: 24486643 Review.
-
Spindle cells and their role in Kaposi's sarcoma.Int J Biochem Cell Biol. 2005 Dec;37(12):2457-65. doi: 10.1016/j.biocel.2005.01.018. Epub 2005 Aug 1. Int J Biochem Cell Biol. 2005. PMID: 16188485 Review.
Cited by
-
Piracy of prostaglandin E2/EP receptor-mediated signaling by Kaposi's sarcoma-associated herpes virus (HHV-8) for latency gene expression: strategy of a successful pathogen.Cancer Res. 2010 May 1;70(9):3697-708. doi: 10.1158/0008-5472.CAN-09-3934. Epub 2010 Apr 13. Cancer Res. 2010. PMID: 20388794 Free PMC article.
-
Current views on the function of the lymphatic vasculature in health and disease.Genes Dev. 2010 Oct 1;24(19):2115-26. doi: 10.1101/gad.1955910. Genes Dev. 2010. PMID: 20889712 Free PMC article. Review.
-
Kaposin-B enhances the PROX1 mRNA stability during lymphatic reprogramming of vascular endothelial cells by Kaposi's sarcoma herpes virus.PLoS Pathog. 2010 Aug 12;6(8):e1001046. doi: 10.1371/journal.ppat.1001046. PLoS Pathog. 2010. PMID: 20730087 Free PMC article.
-
Heterogeneity and plasticity of lymphatic endothelial cells.Semin Thromb Hemost. 2010 Apr;36(3):352-61. doi: 10.1055/s-0030-1253457. Epub 2010 May 20. Semin Thromb Hemost. 2010. PMID: 20490985 Free PMC article. Review.
-
Virus-encoded microRNAs.Virology. 2011 Mar 15;411(2):325-43. doi: 10.1016/j.virol.2011.01.002. Epub 2011 Jan 31. Virology. 2011. PMID: 21277611 Free PMC article. Review.
References
-
- Aluigi MG, Albini A, Carlone S, Repetto L, De Marchi R, Icardi A, Moro M, Noonan D, Benelli R. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi’s sarcoma. Res Virol. 1996;147 (5):267–275. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332 (18):1186–1191. - PubMed
-
- Duensing S, Munger K. Human papillomaviruses and centrosome duplication errors: modeling the origins of genomic instability. Oncogene. 2002;21 (40):6241–6248. - PubMed
-
- Glaunsinger B, Ganem D. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol Cell. 2004;13 (5):713–723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

