Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction
- PMID: 15277218
- PMCID: PMC1618584
- DOI: 10.1016/S0002-9440(10)63309-3
Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction
Abstract
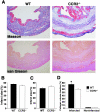
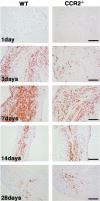
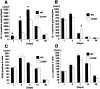
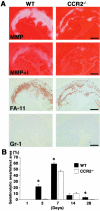
A key component of cardiac remodeling after acute myocardial infarction (MI) is the inflammatory response, which modulates cardiac tissue repair. The purpose of this study was to investigate the relationship between the monocytic inflammatory response and left ventricular remodeling after MI using mice deficient in CC chemokine receptor 2 (CCR2), the primary receptor for the critical regulator of CC chemokine ligand 2. Immunohistochemical analysis revealed rapid infiltration of macrophages into infarcted tissue within 7 days in wild-type (WT) mice. However, this process was greatly impaired in CCR2-deficient (CCR2(-/-)) mice. Echocardiography demonstrated beneficial effects of CCR2 deficiency on left ventricular remodeling at 7 and 28 days after MI. In situ zymography showed augmented gelatinolytic activity in WT mice within 7 days after MI, whereas gelatinolytic activity was barely detectable in CCR2(-/-) mice. Moreover, the distribution of gelatinolytic activity in serial sections was very similar to the distribution of macrophages rather than neutrophils. Expression of matrix metalloproteinases and tumor necrosis factor-alpha mRNAs was up-regulated in infarcted regions from WT mice compared to CCR2(-/-) mice at 3 days after MI. Direct inhibition of CCR2 functional pathway might contribute to the attenuation of left ventricular remodeling after MI.
Figures

Similar articles
-
CC chemokine receptor-2 deficiency attenuates oxidative stress and infarct size caused by myocardial ischemia-reperfusion in mice.Circ J. 2006 Mar;70(3):342-51. doi: 10.1253/circj.70.342. Circ J. 2006. PMID: 16501303
-
Targeted deletion of class A macrophage scavenger receptor increases the risk of cardiac rupture after experimental myocardial infarction.Circulation. 2007 Apr 10;115(14):1904-11. doi: 10.1161/CIRCULATIONAHA.106.671198. Epub 2007 Mar 26. Circulation. 2007. PMID: 17389263
-
C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases.J Pathol. 2004 Dec;204(5):594-604. doi: 10.1002/path.1667. J Pathol. 2004. PMID: 15538737
-
MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy.Inflamm Allergy Drug Targets. 2007 Jun;6(2):101-7. doi: 10.2174/187152807780832265. Inflamm Allergy Drug Targets. 2007. PMID: 17692033 Review.
-
Ventricular remodeling and function: insights using murine echocardiography.J Mol Cell Cardiol. 2010 Mar;48(3):512-7. doi: 10.1016/j.yjmcc.2009.07.004. Epub 2009 Jul 15. J Mol Cell Cardiol. 2010. PMID: 19615377 Free PMC article. Review.
Cited by
-
Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice.Circulation. 2013 May 21;127(20):2038-46. doi: 10.1161/CIRCULATIONAHA.112.000116. Epub 2013 Apr 24. Circulation. 2013. PMID: 23616627 Free PMC article.
-
Role of monocyte chemoattractant protein-1 in myocardial infarction.Int J Biomed Sci. 2007 Sep;3(3):159-67. Int J Biomed Sci. 2007. PMID: 23675039 Free PMC article.
-
Monocytes: protagonists of infarct inflammation and repair after myocardial infarction.Circulation. 2010 Jun 8;121(22):2437-45. doi: 10.1161/CIRCULATIONAHA.109.916346. Circulation. 2010. PMID: 20530020 Free PMC article. Review. No abstract available.
-
Inflammatory cells and their non-coding RNAs as targets for treating myocardial infarction.Basic Res Cardiol. 2018 Dec 6;114(1):4. doi: 10.1007/s00395-018-0712-z. Basic Res Cardiol. 2018. PMID: 30523422 Free PMC article. Review.
-
MCP-1/CCR2 signalling pathway regulates hyperoxia-induced acute lung injury via nitric oxide production.Int J Exp Pathol. 2006 Dec;87(6):475-83. doi: 10.1111/j.1365-2613.2006.00502.x. Int J Exp Pathol. 2006. PMID: 17222215 Free PMC article.
References
-
- Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol. 1991;260:H1406–H1414. - PubMed
-
- Rumberger JA. Ventricular dilatation and remodeling after myocardial infarction. Mayo Clin Proc. 1994;69:664–674. - PubMed
-
- Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89:201–210. - PubMed
-
- Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res. 2000;46:307–315. - PubMed
-
- Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

