6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis
- PMID: 15170386
- PMCID: PMC1133864
- DOI: 10.1042/BJ20040752
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis
Abstract
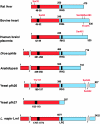
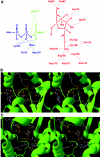
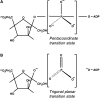
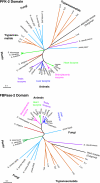
Fru-2,6-P2 (fructose 2,6-bisphosphate) is a signal molecule that controls glycolysis. Since its discovery more than 20 years ago, inroads have been made towards the understanding of the structure-function relationships in PFK-2 (6-phosphofructo-2-kinase)/FBPase-2 (fructose-2,6-bisphosphatase), the homodimeric bifunctional enzyme that catalyses the synthesis and degradation of Fru-2,6-P2. The FBPase-2 domain of the enzyme subunit bears sequence, mechanistic and structural similarity to the histidine phosphatase family of enzymes. The PFK-2 domain was originally thought to resemble bacterial PFK-1 (6-phosphofructo-1-kinase), but this proved not to be correct. Molecular modelling of the PFK-2 domain revealed that, instead, it has the same fold as adenylate kinase. This was confirmed by X-ray crystallography. A PFK-2/FBPase-2 sequence in the genome of one prokaryote, the proteobacterium Desulfovibrio desulfuricans, could be the result of horizontal gene transfer from a eukaryote distantly related to all other organisms, possibly a protist. This, together with the presence of PFK-2/FBPase-2 genes in trypanosomatids (albeit with possibly only one of the domains active), indicates that fusion of genes initially coding for separate PFK-2 and FBPase-2 domains might have occurred early in evolution. In the enzyme homodimer, the PFK-2 domains come together in a head-to-head like fashion, whereas the FBPase-2 domains can function as monomers. There are four PFK-2/FBPase-2 isoenzymes in mammals, each coded by a different gene that expresses several isoforms of each isoenzyme. In these genes, regulatory sequences have been identified which account for their long-term control by hormones and tissue-specific transcription factors. One of these, HNF-6 (hepatocyte nuclear factor-6), was discovered in this way. As to short-term control, the liver isoenzyme is phosphorylated at the N-terminus, adjacent to the PFK-2 domain, by PKA (cAMP-dependent protein kinase), leading to PFK-2 inactivation and FBPase-2 activation. In contrast, the heart isoenzyme is phosphorylated at the C-terminus by several protein kinases in different signalling pathways, resulting in PFK-2 activation.
Figures



Similar articles
-
6-Phosphofructo-2-kinase and fructose-2,6-bisphosphatase in Trypanosomatidae. Molecular characterization, database searches, modelling studies and evolutionary analysis.FEBS J. 2005 Jul;272(14):3542-60. doi: 10.1111/j.1742-4658.2005.04774.x. FEBS J. 2005. PMID: 16008555
-
A role for PFK-2/FBPase-2, as distinct from fructose 2,6-bisphosphate, in regulation of insulin secretion in pancreatic beta-cells.Biochem J. 2008 Apr 1;411(1):41-51. doi: 10.1042/BJ20070962. Biochem J. 2008. PMID: 18039179
-
Cloning and expression in Escherichia coli of a rat hepatoma cell cDNA coding for 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase.Biochem J. 1989 Nov 15;264(1):151-60. doi: 10.1042/bj2640151. Biochem J. 1989. PMID: 2557826 Free PMC article.
-
Evolutionary analysis of fructose 2,6-bisphosphate metabolism.IUBMB Life. 2006 Mar;58(3):133-41. doi: 10.1080/15216540600688280. IUBMB Life. 2006. PMID: 16766380 Review.
-
Rat liver 6-phosphofructo 2-kinase/fructose 2,6-bisphosphatase: a review of relationships between the two activities of the enzyme.J Cell Biochem. 1984;26(1):1-17. doi: 10.1002/jcb.240260102. J Cell Biochem. 1984. PMID: 6096384 Review.
Cited by
-
Emerging Roles and Therapeutic Interventions of Aerobic Glycolysis in Glioma.Onco Targets Ther. 2020 Jul 16;13:6937-6955. doi: 10.2147/OTT.S260376. eCollection 2020. Onco Targets Ther. 2020. PMID: 32764985 Free PMC article. Review.
-
Hypoxic activation of PFKFB4 in breast tumor microenvironment shapes metabolic and cellular plasticity to accentuate metastatic competence.Cell Rep. 2022 Dec 6;41(10):111756. doi: 10.1016/j.celrep.2022.111756. Cell Rep. 2022. PMID: 36476868 Free PMC article.
-
The control systems structures of energy metabolism.J R Soc Interface. 2010 Apr 6;7(45):651-65. doi: 10.1098/rsif.2009.0371. Epub 2009 Oct 14. J R Soc Interface. 2010. PMID: 19828503 Free PMC article.
-
Characterization of the circRNA-miRNA-mRNA Network to Reveal the Potential Functional ceRNAs Associated With Dynamic Changes in the Meat Quality of the Longissimus Thoracis Muscle in Tibetan Sheep at Different Growth Stages.Front Vet Sci. 2022 Apr 1;9:803758. doi: 10.3389/fvets.2022.803758. eCollection 2022. Front Vet Sci. 2022. PMID: 35433904 Free PMC article.
-
Structural and biochemical studies of TIGAR (TP53-induced glycolysis and apoptosis regulator).J Biol Chem. 2009 Jan 16;284(3):1748-54. doi: 10.1074/jbc.M807821200. Epub 2008 Nov 17. J Biol Chem. 2009. PMID: 19015259 Free PMC article.
References
-
- Van Schaftingen E. Fructose 2,6-bisphosphate. Adv. Enzymol. 1987;59:315–395. - PubMed
-
- Pilkis S. J., El-Maghrabi M. R., Claus T. H. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu. Rev. Biochem. 1988;57:755–783. - PubMed
-
- Hue L., Rider M. H., Rousseau G. G. Fructose-2,6-bisphosphate in extra hepatic tissues. In: Pilkis S. J., editor. Fructose-2,6-bisphosphate. Boca Raton: CRC Press; 1990. pp. 173–193.
-
- Van Schaftingen E., Mertens E., Opperdoes F. R. Fructose-2,6-bisphosphate in primitive systems. In: Pilkis S. J., editor. Fructose-2,6-bisphosphate. Boca Raton: CRC Press; 1990. pp. 229–244.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

