Interdependency of fission yeast Alp14/TOG and coiled coil protein Alp7 in microtubule localization and bipolar spindle formation
- PMID: 14742702
- PMCID: PMC379260
- DOI: 10.1091/mbc.e03-11-0837
Interdependency of fission yeast Alp14/TOG and coiled coil protein Alp7 in microtubule localization and bipolar spindle formation
Abstract
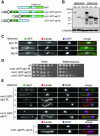
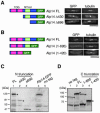
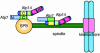
The Dis1/TOG family plays a pivotal role in microtubule organization. In fission yeast, Alp14 and Dis1 share an essential function in bipolar spindle formation. Here, we characterize Alp7, a novel coiled-coil protein that is required for organization of bipolar spindles. Both Alp7 and Alp14 colocalize to the spindle pole body (SPB) and mitotic spindles. Alp14 localization to these sites is fully dependent upon Alp7. Conversely, in the absence of Alp14, Alp7 localizes to the SPBs, but not mitotic spindles. Alp7 forms a complex with Alp14, where the C-terminal region of Alp14 interacts with the coiled-coil domain of Alp7. Intriguingly, this Alp14 C terminus is necessary and sufficient for mitotic spindle localization. Overproduction of either full-length or coiled-coil region of Alp7 results in abnormal V-shaped spindles and stabilization of interphase microtubules, which is induced independent of Alp14. Alp7 may be a functional homologue of animal TACC. Our results shed light on an interdependent relationship between Alp14/TOG and Alp7. We propose a two-step model that accounts for the recruitment of Alp7 and Alp14 to the SPB and microtubules.
Figures


Similar articles
-
Space shuttling in the cell: nucleocytoplasmic transport and microtubule organization during the cell cycle.Nucleus. 2010 May-Jun;1(3):231-6. doi: 10.4161/nucl.1.3.11443. Epub 2010 Feb 8. Nucleus. 2010. PMID: 21327068 Free PMC article. Review.
-
The internal loop of fission yeast Ndc80 binds Alp7/TACC-Alp14/TOG and ensures proper chromosome attachment.Mol Biol Cell. 2013 Apr;24(8):1122-33. doi: 10.1091/mbc.E12-11-0817. Epub 2013 Feb 20. Mol Biol Cell. 2013. PMID: 23427262 Free PMC article.
-
Two XMAP215/TOG Microtubule Polymerases, Alp14 and Dis1, Play Non-Exchangeable, Distinct Roles in Microtubule Organisation in Fission Yeast.Int J Mol Sci. 2019 Oct 15;20(20):5108. doi: 10.3390/ijms20205108. Int J Mol Sci. 2019. PMID: 31618856 Free PMC article.
-
CDK-dependent phosphorylation of Alp7-Alp14 (TACC-TOG) promotes its nuclear accumulation and spindle microtubule assembly.Mol Biol Cell. 2014 Jul 1;25(13):1969-82. doi: 10.1091/mbc.E13-11-0679. Epub 2014 Apr 30. Mol Biol Cell. 2014. PMID: 24790093 Free PMC article.
-
MAPping the eukaryotic tree of life: structure, function, and evolution of the MAP215/Dis1 family of microtubule-associated proteins.Int Rev Cytol. 2004;239:179-272. doi: 10.1016/S0074-7696(04)39004-2. Int Rev Cytol. 2004. PMID: 15464854 Review.
Cited by
-
MAPping the Ndc80 loop in cancer: A possible link between Ndc80/Hec1 overproduction and cancer formation.Bioessays. 2015 Mar;37(3):248-56. doi: 10.1002/bies.201400175. Epub 2015 Jan 2. Bioessays. 2015. PMID: 25557589 Free PMC article. Review.
-
Space shuttling in the cell: nucleocytoplasmic transport and microtubule organization during the cell cycle.Nucleus. 2010 May-Jun;1(3):231-6. doi: 10.4161/nucl.1.3.11443. Epub 2010 Feb 8. Nucleus. 2010. PMID: 21327068 Free PMC article. Review.
-
Alp7/TACC recruits kinesin-8-PP1 to the Ndc80 kinetochore protein for timely mitotic progression and chromosome movement.J Cell Sci. 2015 Jan 15;128(2):354-63. doi: 10.1242/jcs.160036. Epub 2014 Dec 3. J Cell Sci. 2015. PMID: 25472718 Free PMC article.
-
Ndc80 Loop as a protein-protein interaction motif.Cell Div. 2013 Mar 15;8(1):2. doi: 10.1186/1747-1028-8-2. Cell Div. 2013. PMID: 23497645 Free PMC article.
-
Microtubule polymerase and processive plus-end tracking functions originate from distinct features within TOG domain arrays.Mol Biol Cell. 2019 Jun 1;30(12):1490-1504. doi: 10.1091/mbc.E19-02-0093. Epub 2019 Apr 10. Mol Biol Cell. 2019. PMID: 30969896 Free PMC article.
References
-
- Bähler, J., Wu, J., Longtine, M.S., Shah, N.G., McKenzie III, A., Steever, A.B., Wach, A., Philippsen, P., and Pringle, J.R. (1998). Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943-951. - PubMed
-
- Bellanger, J.-M., and Gönczy, P. (2003). TAC-1 and ZYG-9 form a complex that promotes microtubule assembly in C. elegans embryos. Curr. Biol. 13, 1488-1498. - PubMed
-
- Cullen, C.F., and Ohkura, H. (2001). Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat. Cell Biol. 3, 637-642. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

