Differential efficacy of caspase inhibitors on apoptosis markers during sepsis in rats and implication for fractional inhibition requirements for therapeutics
- PMID: 14718517
- PMCID: PMC2211770
- DOI: 10.1084/jem.20031791
Differential efficacy of caspase inhibitors on apoptosis markers during sepsis in rats and implication for fractional inhibition requirements for therapeutics
Abstract
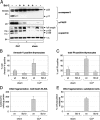
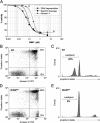
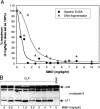
A rodent model of sepsis was used to establish the relationship between caspase inhibition and inhibition of apoptotic cell death in vivo. In this model, thymocyte cell death was blocked by Bcl-2 transgene, indicating that apoptosis was predominantly dependent on the mitochondrial pathway that culminates in caspase-3 activation. Caspase inhibitors, including the selective caspase-3 inhibitor M867, were able to block apoptotic manifestations both in vitro and in vivo but with strikingly different efficacy for different cell death markers. Inhibition of DNA fragmentation required substantially higher levels of caspase-3 attenuation than that required for blockade of other apoptotic events such as spectrin proteolysis and phosphatidylserine externalization. These data indicate a direct relationship between caspase inhibition and some apoptotic manifestations but that small quantities of uninhibited caspase-3 suffice to initiate genomic DNA breakdown, presumably through the escape of catalytic quantities of caspase-activated DNase. These findings suggest that putative caspase-independent apoptosis may be overestimated in some systems since blockade of spectrin proteolysis and other cell death markers does not accurately reflect the high degrees of caspase-3 inhibition needed to prevent DNA fragmentation. Furthermore, this requirement presents substantial therapeutic challenges owing to the need for persistent and complete caspase blockade.
Figures

Similar articles
-
Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte.Nat Immunol. 2000 Dec;1(6):496-501. doi: 10.1038/82741. Nat Immunol. 2000. PMID: 11101871
-
M867, a novel selective inhibitor of caspase-3 enhances cell death and extends tumor growth delay in irradiated lung cancer models.PLoS One. 2008 May 28;3(5):e2275. doi: 10.1371/journal.pone.0002275. PLoS One. 2008. PMID: 18509530 Free PMC article.
-
Cytotoxicity of diacetoxyscirpenol is associated with apoptosis by activation of caspase-8 and interruption of cell cycle progression by down-regulation of cdk4 and cyclin B1 in human Jurkat T cells.Toxicol Appl Pharmacol. 2007 Jul 15;222(2):190-201. doi: 10.1016/j.taap.2007.04.011. Epub 2007 May 8. Toxicol Appl Pharmacol. 2007. PMID: 17559898
-
Targeting inflammatory diseases via apoptotic mechanisms.Curr Opin Pharmacol. 2003 Aug;3(4):412-9. doi: 10.1016/s1471-4892(03)00072-9. Curr Opin Pharmacol. 2003. PMID: 12901951 Review.
-
Caspase inhibitors: a pharmaceutical industry perspective.Curr Top Med Chem. 2005;5(16):1697-717. doi: 10.2174/156802605775009720. Curr Top Med Chem. 2005. PMID: 16375749 Review.
Cited by
-
Inhibition of Apoptosis in a Model of Ischemic Stroke Leads to Enhanced Cell Survival, Endogenous Neural Precursor Cell Activation and Improved Functional Outcomes.Int J Mol Sci. 2024 Feb 1;25(3):1786. doi: 10.3390/ijms25031786. Int J Mol Sci. 2024. PMID: 38339065 Free PMC article.
-
The roles of intracellular proteolysis in cardiac ischemia-reperfusion injury.Basic Res Cardiol. 2023 Sep 28;118(1):38. doi: 10.1007/s00395-023-01007-z. Basic Res Cardiol. 2023. PMID: 37768438 Review.
-
Intracellular and Extracellular Lipopolysaccharide Signaling in Sepsis: Avenues for Novel Therapeutic Strategies.J Innate Immun. 2021;13(6):323-332. doi: 10.1159/000515740. Epub 2021 May 18. J Innate Immun. 2021. PMID: 34004605 Free PMC article. Review.
-
Dysregulated myelopoiesis and hematopoietic function following acute physiologic insult.Curr Opin Hematol. 2018 Jan;25(1):37-43. doi: 10.1097/MOH.0000000000000395. Curr Opin Hematol. 2018. PMID: 29035909 Free PMC article. Review.
-
Caspase vinyl sulfone small molecule inhibitors prevent axonal degeneration in human neurons and reverse cognitive impairment in Caspase-6-overexpressing mice.Mol Neurodegener. 2017 Feb 28;12(1):22. doi: 10.1186/s13024-017-0166-z. Mol Neurodegener. 2017. PMID: 28241839 Free PMC article.
References
-
- Nicholson, D.W. 1999. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6:1028–1042. - PubMed
-
- Shi, Y. 2002. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell. 9:459–470. - PubMed
-
- Slee, E.A., C. Adrain, and S.J. Martin. 2001. Executioner caspase-3, -6 and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 276:7320–7326. - PubMed
-
- Kuida, K., T.S. Zheng, S. Na, C. Kuan, D. Yang, H. Karasumaya, P. Rakik, and R.A. Flavell. 1996. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 384:368–372. - PubMed
-
- Thornberry, N.A., and Y. Lazbenik. 1998. Caspases: enemies within. Science. 281:1312–1316. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

