Role of the ectodomain of the gp41 transmembrane envelope protein of human immunodeficiency virus type 1 in late steps of the membrane fusion process
- PMID: 14694113
- PMCID: PMC368777
- DOI: 10.1128/jvi.78.2.811-820.2004
Role of the ectodomain of the gp41 transmembrane envelope protein of human immunodeficiency virus type 1 in late steps of the membrane fusion process
Abstract
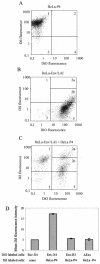
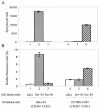
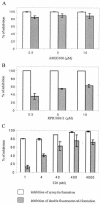
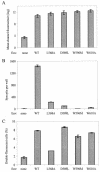
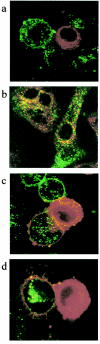
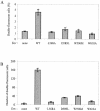
The membrane fusion process mediated by the gp41 transmembrane envelope glycoprotein of the human immunodeficiency virus type 1 (HIV-1) was addressed by a flow cytometry assay detecting exchanges of fluorescent membrane probes (DiI and DiO) between cells expressing the HIV-1 envelope proteins (Env) and target cells. Double-fluorescent cells were detected when target cells expressed the type of chemokine receptor, CXCR4 or CCR5, matching the type of gp120 surface envelope protein, X4 or R5, respectively. Background levels of double-fluorescent cells were observed when the gp120-receptor interaction was blocked by AMD3100, a CXCR4 antagonist. The L568A mutation in the N-terminal heptad repeat (HR1) of gp41 resulted in parallel inhibition of the formation of syncytia and double-fluorescent cells, indicating that gp41 had a direct role in the exchange of fluorescent probes. In contrast, three mutations in the loop region of the gp41 ectodomain, located on either side of the Cys-(X)(5)-Cys motif (W596 M and W610A) or at the distal end of HR1 (D589L), had limited or no apparent effect on membrane lipid mixing between Env(+) and target cells, while they blocked formation of syncytia and markedly reduced the exchanges of cytoplasmic fluorescent probes. The loop region could therefore have a direct or indirect role in events occurring after the merging of membranes, such as the formation or dilation of fusion pores. Two types of inhibitors of HIV-1 entry, the gp41-derived peptide T20 and the betulinic acid derivative RPR103611, had limited effects on membrane exchanges at concentrations blocking or markedly reducing syncytium formation. This finding confirmed that T20 can inhibit the late steps of membrane fusion (post-lipid mixing) and brought forth an indirect argument for the role of the gp41 loop region in these steps, as mutations conferring resistance to RPR103611V were mapped in this region (I595S or L602H).
Figures

Similar articles
-
Sensitivity to a nonpeptidic compound (RPR103611) blocking human immunodeficiency virus type 1 Env-mediated fusion depends on sequence and accessibility of the gp41 loop region.J Virol. 2000 Mar;74(5):2142-50. doi: 10.1128/jvi.74.5.2142-2150.2000. J Virol. 2000. PMID: 10666243 Free PMC article.
-
Mutations That Increase the Stability of the Postfusion gp41 Conformation of the HIV-1 Envelope Glycoprotein Are Selected by both an X4 and R5 HIV-1 Virus To Escape Fusion Inhibitors Corresponding to Heptad Repeat 1 of gp41, but the gp120 Adaptive Mutations Differ between the Two Viruses.J Virol. 2019 May 15;93(11):e00142-19. doi: 10.1128/JVI.00142-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30894471 Free PMC article.
-
Resistance to a drug blocking human immunodeficiency virus type 1 entry (RPR103611) is conferred by mutations in gp41.J Virol. 1997 Nov;71(11):8230-6. doi: 10.1128/JVI.71.11.8230-8236.1997. J Virol. 1997. PMID: 9343174 Free PMC article.
-
Biochemistry and biophysics of HIV-1 gp41 - membrane interactions and implications for HIV-1 envelope protein mediated viral-cell fusion and fusion inhibitor design.Curr Top Med Chem. 2011 Dec;11(24):2959-84. doi: 10.2174/156802611798808497. Curr Top Med Chem. 2011. PMID: 22044229 Free PMC article. Review.
-
HIV-1 gp41: mediator of fusion and target for inhibition.AIDS Rev. 2003 Oct-Dec;5(4):214-21. AIDS Rev. 2003. PMID: 15012000 Review.
Cited by
-
Active Components from Cassia abbreviata Prevent HIV-1 Entry by Distinct Mechanisms of Action.Int J Mol Sci. 2021 May 10;22(9):5052. doi: 10.3390/ijms22095052. Int J Mol Sci. 2021. PMID: 34068829 Free PMC article.
-
A low-molecular-weight entry inhibitor of both CCR5- and CXCR4-tropic strains of human immunodeficiency virus type 1 targets a novel site on gp41.J Virol. 2010 Jul;84(14):7288-99. doi: 10.1128/JVI.00535-10. Epub 2010 Apr 28. J Virol. 2010. PMID: 20427524 Free PMC article.
-
HIV entry and envelope glycoprotein-mediated fusion.J Biol Chem. 2012 Nov 30;287(49):40841-9. doi: 10.1074/jbc.R112.406272. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043104 Free PMC article. Review.
-
Antiviral properties of two trimeric recombinant gp41 proteins.Retrovirology. 2006 Mar 3;3:16. doi: 10.1186/1742-4690-3-16. Retrovirology. 2006. PMID: 16515685 Free PMC article.
-
Structural basis of antiviral activity of peptides from MPER of FIV gp36.PLoS One. 2018 Sep 21;13(9):e0204042. doi: 10.1371/journal.pone.0204042. eCollection 2018. PLoS One. 2018. PMID: 30240422 Free PMC article.
References
-
- Baker, K. A., R. E. Dutch, R. A. Lamb, and T. S. Jardetzky. 1999. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell 3:309-319. - PubMed
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
-
- Blumenthal, R., M. J. Clague, S. R. Durell, and R. M. Epand. 2003. Membrane fusion. Chem. Rev. 103:53-69. - PubMed
-
- Brelot, A., and M. Alizon. 2001. HIV-1 entry and how to block it. AIDS 15:S3-S11. - PubMed
-
- Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

