Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence
- PMID: 14657224
- PMCID: PMC2194133
- DOI: 10.1084/jem.20022227
Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence
Abstract
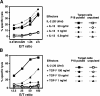
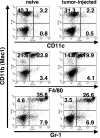
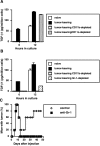
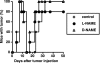
Our previous work demonstrated that cytotoxic T lymphocyte (CTL)-mediated tumor immunosurveillance of the 15-12RM tumor could be suppressed by a CD1d-restricted lymphocyte, most likely a natural killer (NK) T cell, which produces interleukin (IL)-13. Here we present evidence for the effector elements in this suppressive pathway. T cell-reconstituted recombination activating gene (RAG)2 knockout (KO) and RAG2/IL-4 receptor alpha double KO mice showed that inhibition of immunosurveillance requires IL-13 responsiveness by a non-T non-B cell. Such nonlymphoid splenocytes from tumor-bearing mice produced more transforming growth factor (TGF)-beta, a potent inhibitor of CTL, ex vivo than such cells from naive mice, and this TGF-beta production was dependent on the presence in vivo of both IL-13 and CD1d-restricted T cells. Ex vivo TGF-beta production was also abrogated by depleting either CD11b+ or Gr-1+ cells from the nonlymphoid cells of tumor-bearing mice. Further, blocking TGF-beta or depleting Gr-1+ cells in vivo prevented the tumor recurrence, implying that TGF-beta made by a CD11b+ Gr-1+ myeloid cell, in an IL-13 and CD1d-restricted T cell-dependent mechanism, is necessary for down-regulation of tumor immunosurveillance. Identification of this stepwise regulation of immunosurveillance, involving CD1-restricted T cells, IL-13, myeloid cells, and TGF-beta, explains previous observations on myeloid suppressor cells or TGF-beta and provides insights for targeted approaches for cancer immunotherapy, including synergistic blockade of TGF-beta and IL-13.
Figures


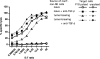
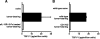


Similar articles
-
CD1d-restricted natural killer T cells can down-regulate tumor immunosurveillance independent of interleukin-4 receptor-signal transducer and activator of transcription 6 or transforming growth factor-beta.Cancer Res. 2006 Apr 1;66(7):3869-75. doi: 10.1158/0008-5472.CAN-05-3421. Cancer Res. 2006. PMID: 16585215
-
NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway.Nat Immunol. 2000 Dec;1(6):515-20. doi: 10.1038/82771. Nat Immunol. 2000. PMID: 11101874
-
Inhibition of tumor-specific cytotoxic T-lymphocyte responses by transforming growth factor beta 1.Cancer Res. 1992 Mar 15;52(6):1386-92. Cancer Res. 1992. PMID: 1531782
-
CD1d-restricted "NKT" cells and myeloid IL-12 production: an immunological crossroads leading to promotion or suppression of effective anti-tumor immune responses?J Leukoc Biol. 2004 Aug;76(2):307-13. doi: 10.1189/jlb.0104038. Epub 2004 May 3. J Leukoc Biol. 2004. PMID: 15123775 Review.
-
Signal transducer and activator of transcription 6 (Stat6) and CD1: inhibitors of immunosurveillance against primary tumors and metastatic disease.Cancer Immunol Immunother. 2004 Feb;53(2):86-91. doi: 10.1007/s00262-003-0446-z. Epub 2003 Oct 30. Cancer Immunol Immunother. 2004. PMID: 14593499 Free PMC article. Review.
Cited by
-
Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation.Front Immunol. 2020 Jul 22;11:1371. doi: 10.3389/fimmu.2020.01371. eCollection 2020. Front Immunol. 2020. PMID: 32793192 Free PMC article. Review.
-
A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers.Clin Cancer Res. 2010 Sep 15;16(18):4583-94. doi: 10.1158/1078-0432.CCR-10-0733. Epub 2010 Aug 11. Clin Cancer Res. 2010. PMID: 20702612 Free PMC article.
-
Interferon regulatory factor-8 modulates the development of tumour-induced CD11b+Gr-1+ myeloid cells.J Cell Mol Med. 2009 Sep;13(9B):3939-50. doi: 10.1111/j.1582-4934.2009.00685.x. J Cell Mol Med. 2009. PMID: 20196788 Free PMC article.
-
The role of NKT cells in gastrointestinal cancers.Oncoimmunology. 2021 Dec 30;11(1):2009666. doi: 10.1080/2162402X.2021.2009666. eCollection 2022. Oncoimmunology. 2021. PMID: 36524208 Free PMC article. Review.
-
Going both ways: immune regulation via CD1d-dependent NKT cells.J Clin Invest. 2004 Nov;114(10):1379-88. doi: 10.1172/JCI23594. J Clin Invest. 2004. PMID: 15545985 Free PMC article. Review.
References
-
- Dunn, G.P., A.T. Bruce, H. Ikeda, L.J. Old, and R.D. Schreiber. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3:991–998. - PubMed
-
- Matsui, S., J.D. Ahlers, A.O. Vortmeyer, M. Terabe, T. Tsukui, D.P. Carbone, L.A. Liotta, and J. Berzofsky. 1999. A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J. Immunol. 163:184–193. - PubMed
-
- Terabe, M., S. Matsui, N. Noben-Trauth, H. Chen, C. Watson, D.D. Donaldson, D.P. Carbone, W.E. Paul, and J.A. Berzofsky. 2000. NKT cell-mediated repression of tumour immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 1:515–520. - PubMed
-
- Ostrand-Rosenberg, S., M.J. Grusby, and V.K. Clements. 2000. Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. J. Immunol. 165:6015–6019. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

