Chk1 kinase negatively regulates mitotic function of Cdc25A phosphatase through 14-3-3 binding
- PMID: 14559997
- PMCID: PMC207598
- DOI: 10.1128/MCB.23.21.7488-7497.2003
Chk1 kinase negatively regulates mitotic function of Cdc25A phosphatase through 14-3-3 binding
Abstract
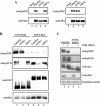
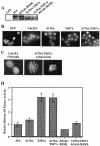
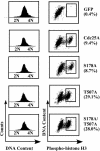
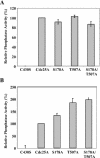
The order and fidelity of cell cycle events in mammals is intimately linked to the integrity of the Chk1 kinase-Cdc25A phosphatase pathway. Chk1 phosphorylation targets Cdc25A for destruction and, as shown here, inhibits interactions between Cdc25A and its mitotic substrate cyclin B1-Cdk1. Phosphorylation of Cdc25A on serine 178 and threonine 507 facilitates 14-3-3 binding, and Chk1 phosphorylates both residues in vitro. Mutation of T507 to alanine (T507A) enhanced the biological activity of Cdc25A. Cdc25A(T507A) was more efficient in binding to cyclin B1, activating cyclin B1-Cdk1, and promoting premature entry into mitosis. We propose that the Chk1/Cdc25A/14-3-3 pathway functions to prevent cells from entering into mitosis prior to replicating their genomes to ensure the fidelity of the cell division process.
Figures




Similar articles
-
Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25.Science. 1997 Sep 5;277(5331):1497-501. doi: 10.1126/science.277.5331.1497. Science. 1997. PMID: 9278511
-
Chk1, but not Chk2, inhibits Cdc25 phosphatases by a novel common mechanism.EMBO J. 2004 Aug 18;23(16):3386-96. doi: 10.1038/sj.emboj.7600328. Epub 2004 Jul 22. EMBO J. 2004. PMID: 15272308 Free PMC article.
-
Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1.Nature. 1998 Oct 1;395(6701):507-10. doi: 10.1038/26766. Nature. 1998. PMID: 9774107
-
Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C.Curr Opin Cell Biol. 2000 Dec;12(6):658-65. doi: 10.1016/s0955-0674(00)00149-6. Curr Opin Cell Biol. 2000. PMID: 11063929 Review.
-
Cell cycle regulation by the Cdc25 phosphatase family.Prog Cell Cycle Res. 2000;4:107-14. doi: 10.1007/978-1-4615-4253-7_10. Prog Cell Cycle Res. 2000. PMID: 10740819 Review.
Cited by
-
Negative Charges, Not Necessary Phosphorylation, are Required for Ligand Recognition by 14-3-3 Proteins.bioRxiv [Preprint]. 2024 Sep 19:2024.09.16.613320. doi: 10.1101/2024.09.16.613320. bioRxiv. 2024. PMID: 39345434 Free PMC article. Preprint.
-
Cryo-EM structure of the CDK2-cyclin A-CDC25A complex.Nat Commun. 2024 Aug 9;15(1):6807. doi: 10.1038/s41467-024-51135-w. Nat Commun. 2024. PMID: 39122719 Free PMC article.
-
The Development of CDC25A-Derived Phosphoseryl Peptides That Bind 14-3-3ε with High Affinities.Int J Mol Sci. 2024 Apr 30;25(9):4918. doi: 10.3390/ijms25094918. Int J Mol Sci. 2024. PMID: 38732131 Free PMC article.
-
14-3-3ε: a protein with complex physiology function but promising therapeutic potential in cancer.Cell Commun Signal. 2024 Jan 26;22(1):72. doi: 10.1186/s12964-023-01420-w. Cell Commun Signal. 2024. PMID: 38279176 Free PMC article. Review.
-
Optimizing Phosphopeptide Structures That Target 14-3-3ε in Cutaneous Squamous Cell Carcinoma.ACS Omega. 2024 Jan 3;9(2):2719-2729. doi: 10.1021/acsomega.3c07740. eCollection 2024 Jan 16. ACS Omega. 2024. PMID: 38250398 Free PMC article.
References
-
- Bartek, J., and J. Lukas. 2001. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 490:117-122. - PubMed
-
- Bernardi, R., D. A. Lieberman, and B. Hoffman. 2000. Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene 19:2447-2454. - PubMed
-
- Broggini, M., G. Buraggi, A. Brenna, L. Riva, A. Codegoni, V. Torri, A. Lissoni, C. Mangioni, and M. D'Incalci. 2000. Cell cycle-related phosphatases Cdc25A and B expression correlates with survival in ovarian cancer patients. Anticancer Res. 20:4835-4840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
