c-Myc augments gamma irradiation-induced apoptosis by suppressing Bcl-XL
- PMID: 14517295
- PMCID: PMC230315
- DOI: 10.1128/MCB.23.20.7256-7270.2003
c-Myc augments gamma irradiation-induced apoptosis by suppressing Bcl-XL
Abstract
Alterations in MYC and p53 are hallmarks of cancer. p53 coordinates the response to gamma irradiation (gamma-IR) by either triggering apoptosis or cell cycle arrest. c-Myc activates the p53 apoptotic checkpoint, and thus tumors overexpressing MYC often harbor p53 mutations. Nonetheless, many of these cancers are responsive to therapy, suggesting that Myc may sensitize cells to gamma-IR independent of p53. In mouse embryo fibroblasts (MEFs) and in E micro -myc transgenic B cells in vivo, c-Myc acts in synergy with gamma-IR to trigger apoptosis, but alone, when cultured in growth medium, it does not induce a DNA damage response. Surprisingly, c-Myc also sensitizes p53-deficient MEFs to gamma-IR-induced apoptosis. In normal cells, and in precancerous B cells of E micro -myc transgenic mice, this apoptotic response is associated with the suppression of the antiapoptotic regulators Bcl-2 and Bcl-X(L) and with the concomitant induction of Puma, a proapoptotic BH3-only protein. However, in p53-null MEFs only Bcl-X(L) expression was suppressed, suggesting levels of Bcl-X(L) regulate the response to gamma-IR. Indeed, Bcl-X(L) overexpression blocked this apoptotic response, whereas bcl-X-deficient MEFs were inherently and selectively sensitive to gamma-IR-induced apoptosis. Therefore, MYC may sensitize tumor cells to DNA damage by suppressing Bcl-X.
Figures
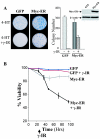
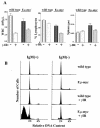
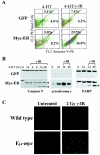
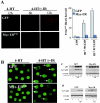
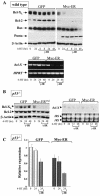
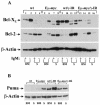
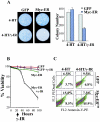
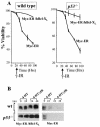
Similar articles
-
Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53.J Biol Chem. 2006 Mar 17;281(11):7260-70. doi: 10.1074/jbc.M509868200. Epub 2006 Jan 6. J Biol Chem. 2006. PMID: 16407291
-
Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis.Mol Cell Biol. 2001 Aug;21(15):5063-70. doi: 10.1128/MCB.21.15.5063-5070.2001. Mol Cell Biol. 2001. PMID: 11438662 Free PMC article.
-
Combined loss of PUMA and p21 accelerates c-MYC-driven lymphoma development considerably less than loss of one allele of p53.Oncogene. 2016 Jul 21;35(29):3866-71. doi: 10.1038/onc.2015.457. Epub 2015 Dec 7. Oncogene. 2016. PMID: 26640149
-
No PUMA, no death: implications for p53-dependent apoptosis.Cancer Cell. 2003 Oct;4(4):248-9. doi: 10.1016/s1535-6108(03)00249-6. Cancer Cell. 2003. PMID: 14585351 Review.
-
BCL2 family in DNA damage and cell cycle control.Cell Death Differ. 2006 Aug;13(8):1351-9. doi: 10.1038/sj.cdd.4401987. Epub 2006 Jun 9. Cell Death Differ. 2006. PMID: 16763616 Review.
Cited by
-
Myc is required for activation of the ATM-dependent checkpoints in response to DNA damage.PLoS One. 2010 Jan 27;5(1):e8924. doi: 10.1371/journal.pone.0008924. PLoS One. 2010. PMID: 20111719 Free PMC article.
-
Hypoxia-induced decrease in p53 protein level and increase in c-jun DNA binding activity results in cancer cell resistance to etoposide.Neoplasia. 2009 Oct;11(10):976-86. doi: 10.1593/neo.09632. Neoplasia. 2009. PMID: 19794957 Free PMC article.
-
Apoptosis regulation in adrenocortical carcinoma.Endocr Connect. 2019 May 1;8(5):R91-R104. doi: 10.1530/EC-19-0114. Endocr Connect. 2019. PMID: 30978697 Free PMC article. Review.
-
Myc is involved in Genistein protecting against LPS-induced myocarditis in vitro through mediating MAPK/JNK signaling pathway.Biosci Rep. 2020 Jun 26;40(6):BSR20194472. doi: 10.1042/BSR20194472. Biosci Rep. 2020. PMID: 32515469 Free PMC article.
-
BIM is the primary mediator of MYC-induced apoptosis in multiple solid tissues.Cell Rep. 2014 Sep 11;8(5):1347-53. doi: 10.1016/j.celrep.2014.07.057. Epub 2014 Aug 28. Cell Rep. 2014. PMID: 25176652 Free PMC article.
References
-
- Adams, J. M., A. W. Harris, C. A. Pinkert, L. M. Corcoran, W. S. Alexander, S. Cory, R. D. Palmiter, and R. L. Brinster. 1985. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318:533-538. - PubMed
-
- Askew, D. S., R. A. Ashmun, B. C. Simmons, and J. L. Cleveland. 1991. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene 6:1915-1922. - PubMed
-
- Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
