The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12
- PMID: 12953097
- PMCID: PMC2194187
- DOI: 10.1084/jem.20020437
The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12
Abstract
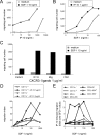
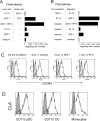
The recruitment of selected dendritic cell (DC) subtypes conditions the class of the immune response. Here we show that the migration of human plasmacytoid DCs (pDCs), the blood natural interferon alpha-producing cells, is induced upon the collective action of inducible and constitutive chemokines. Despite expression of very high levels of CXCR3, pDCs do not respond efficiently to CXCR3 ligands. However, they migrate in response to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12 and CXCR3 ligands synergize with SDF-1/CXCL12 to induce pDC migration. This synergy reflects a sensitizing effect of CXCR3 ligands, which, independently of a gradient and chemoattraction, decrease by 20-50-fold the threshold of sensitivity to SDF-1/CXCL12. Thus, the ability of the constitutive chemokine SDF-1/CXCL12 to induce pDC recruitment might be controlled by CXCR3 ligands released during inflammation such as in virus infection. SDF-1/CXCL12 and the CXCR3 ligands Mig/CXCL9 and ITAC/CXCL1 display adjacent expression both in secondary lymphoid organs and in inflamed epithelium from virus-induced pathologic lesions. Because pDCs express both the lymph node homing molecule l-selectin and the cutaneous homing molecule cutaneous lymphocyte antigen, the cooperation between inducible CXCR3 ligands and constitutive SDF-1/CXCL12 may regulate recruitment of pDCs either in lymph nodes or at peripheral sites of inflammation.
Figures




Similar articles
-
In situ leukemic plasmacytoid dendritic cells pattern of chemokine receptors expression and in vitro migratory response.Leukemia. 2004 Sep;18(9):1491-8. doi: 10.1038/sj.leu.2403452. Leukemia. 2004. PMID: 15284853
-
Plasmacytoid dendritic cell recruitment by immobilized CXCR3 ligands.J Immunol. 2004 Dec 1;173(11):6592-602. doi: 10.4049/jimmunol.173.11.6592. J Immunol. 2004. PMID: 15557149
-
Ultraviolet radiation-induced injury, chemokines, and leukocyte recruitment: An amplification cycle triggering cutaneous lupus erythematosus.Arthritis Rheum. 2005 May;52(5):1504-16. doi: 10.1002/art.21034. Arthritis Rheum. 2005. PMID: 15880822
-
Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells.Hum Immunol. 2002 Dec;63(12):1164-71. doi: 10.1016/s0198-8859(02)00755-3. Hum Immunol. 2002. PMID: 12480260 Review.
-
MIG in psoriatic arthritis.Clin Ter. 2018 Nov-Dec;169(6):e297-e302. doi: 10.7417/CT.2018.2097. Clin Ter. 2018. PMID: 30554252 Review.
Cited by
-
Plasmacytoid dendritic cells and their therapeutic activity in cancer.Oncoimmunology. 2012 Aug 1;1(5):726-734. doi: 10.4161/onci.20171. Oncoimmunology. 2012. PMID: 22934264 Free PMC article.
-
Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin.J Exp Med. 2005 Feb 21;201(4):509-15. doi: 10.1084/jem.20041310. J Exp Med. 2005. PMID: 15728234 Free PMC article.
-
Heterodimers Are an Integral Component of Chemokine Signaling Repertoire.Int J Mol Sci. 2023 Jul 19;24(14):11639. doi: 10.3390/ijms241411639. Int J Mol Sci. 2023. PMID: 37511398 Free PMC article. Review.
-
Glycosaminoglycan Interactions with Chemokines Add Complexity to a Complex System.Pharmaceuticals (Basel). 2017 Aug 9;10(3):70. doi: 10.3390/ph10030070. Pharmaceuticals (Basel). 2017. PMID: 28792472 Free PMC article. Review.
-
Modulation of Chemokine Responses: Synergy and Cooperativity.Front Immunol. 2016 May 19;7:183. doi: 10.3389/fimmu.2016.00183. eCollection 2016. Front Immunol. 2016. PMID: 27242790 Free PMC article. Review.
References
-
- Galibert, L., C.R. Maliszewski, and S. Vandenabeele. 2001. Plasmacytoid monocytes/T cells: a dendritic cell lineage? Semin. Immunol. 13:283–289. - PubMed
-
- Res, P.C., F. Couwenberg, F.A. Vyth-Dreese, and H. Spits. 1999. Expression of pTalpha mRNA in a committed dendritic cell precursor in the human thymus. Blood. 94:2647–2657. - PubMed
-
- Siegal, F.P., N. Kadowaki, M. Shodell, P.A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. - PubMed
-
- Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919–923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

