The virus-encoded chemokine vMIP-II inhibits virus-induced Tc1-driven inflammation
- PMID: 12805438
- PMCID: PMC164793
- DOI: 10.1128/jvi.77.13.7393-7400.2003
The virus-encoded chemokine vMIP-II inhibits virus-induced Tc1-driven inflammation
Abstract
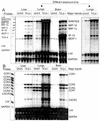
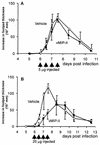
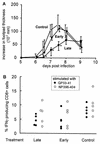
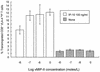
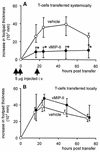
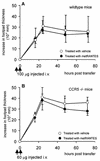
The human herpesvirus 8-encoded protein vMIP-II is a potent in vitro antagonist of many chemokine receptors believed to be associated with attraction of T cells with a type 1 cytokine profile. For the present report we have studied the in vivo potential of this viral chemokine antagonist to inhibit virus-induced T-cell-mediated inflammation. This was done by use of the well-established model system murine lymphocytic choriomeningitis virus infection. Mice were infected in the footpad, and the induced CD8(+) T-cell-dependent inflammation was evaluated in mice subjected to treatment with vMIP-II. We found that inflammation was markedly inhibited in mice treated during the efferent phase of the antiviral immune response. In vitro studies revealed that vMIP-II inhibited chemokine-induced migration of activated CD8(+) T cells, but not T-cell-target cell contact, granule exocytosis, or cytokine release. Consistent with these in vitro findings treatment with vMIP-II inhibited the adoptive transfer of a virus-specific delayed-type hypersensitivity response in vivo, but only when antigen-primed donor cells were transferred via the intravenous route and required to migrate actively, not when the cells were injected directly into the test site. In contrast to the marked inhibition of the effector phase, the presence of vMIP-II during the afferent phase of the immune response did not result in significant suppression of virus-induced inflammation. Taken together, these results indicate that chemokine-induced signals are pivotal in directing antiviral effector cells toward virus-infected organ sites and that vMIP-II is a potent inhibitor of type 1 T-cell-mediated inflammation.
Figures

Similar articles
-
CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system.J Immunol. 2006 Apr 1;176(7):4235-43. doi: 10.4049/jimmunol.176.7.4235. J Immunol. 2006. PMID: 16547260
-
Alpha 4 integrin directs virus-activated CD8+ T cells to sites of infection.J Immunol. 1995 May 15;154(10):5293-301. J Immunol. 1995. PMID: 7537304
-
Eukaryotic expression of the broad-spectrum chemokine receptor antagonist vMIP-II and its effects on T-cell function in vitro and in vivo.Exp Dermatol. 2006 Aug;15(8):634-42. doi: 10.1111/j.1600-0625.2006.00455.x. Exp Dermatol. 2006. PMID: 16842602
-
Host factors influencing viral persistence.Philos Trans R Soc Lond B Biol Sci. 2000 Aug 29;355(1400):1031-41. doi: 10.1098/rstb.2000.0640. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11186304 Free PMC article. Review.
-
Biology and pathogenesis of lymphocytic choriomeningitis virus infection.Curr Top Microbiol Immunol. 2002;263:83-117. doi: 10.1007/978-3-642-56055-2_6. Curr Top Microbiol Immunol. 2002. PMID: 11987822 Review. No abstract available.
Cited by
-
Tumor immune escape by the loss of homeostatic chemokine expression.Proc Natl Acad Sci U S A. 2007 Nov 27;104(48):19055-60. doi: 10.1073/pnas.0705673104. Epub 2007 Nov 19. Proc Natl Acad Sci U S A. 2007. PMID: 18025475 Free PMC article.
-
Novel Bivalent and D-Peptide Ligands of CXCR4 Mobilize Hematopoietic Progenitor Cells to the Blood in C3H/HeJ Mice.Cell Transplant. 2018 Aug;27(8):1249-1255. doi: 10.1177/0963689718784957. Epub 2018 Jul 11. Cell Transplant. 2018. PMID: 29991278 Free PMC article.
-
CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands.Int J Mol Sci. 2020 Oct 15;21(20):7619. doi: 10.3390/ijms21207619. Int J Mol Sci. 2020. PMID: 33076281 Free PMC article. Review.
-
Inflammation, immunity and potential target therapy of SARS-COV-2: A total scale analysis review.Food Chem Toxicol. 2021 Apr;150:112087. doi: 10.1016/j.fct.2021.112087. Epub 2021 Feb 25. Food Chem Toxicol. 2021. PMID: 33640537 Free PMC article. Review.
-
Lymphocytic choriomeningitis virus-induced central nervous system disease: a model for studying the role of chemokines in regulating the acute antiviral CD8+ T-cell response in an immune-privileged organ.J Virol. 2009 Jan;83(1):20-8. doi: 10.1128/JVI.00682-08. Epub 2008 Sep 10. J Virol. 2009. PMID: 18787010 Free PMC article. Review. No abstract available.
References
-
- Andersson, E. C., J. P. Christensen, O. Marker, and A. R. Thomsen. 1994. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J. Immunol. 152:1237-1245. - PubMed
-
- Andreasen, S. O., J. E. Christensen, O. Marker, and A. R. Thomsen. 2000. Role of CD40 ligand and CD28 in induction and maintenance of antiviral CD8+ effector T cell responses. J. Immunol. 164:3689-3697. - PubMed
-
- Andreasen, S. O., J. P. Christensen, O. Marker, and A. R. Thomsen. 1999. Virus-induced non-specific signals cause cell cycle progression of primed CD8+ T cells but do not induce cell differentiation. Int. Immunol. 11:1463-1473. - PubMed
-
- Boshoff, C., Y. Endo, P. D. Collins, Y. Takeuchi, J. D. Reeves, V. L. Schweickart, M. A. Siani, T. Sasaki, T. J. Williams, P. W. Gray, P. S. Moore, Y. Chang, and R. A. Weiss. 1997. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science 278:290-294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

