NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis
- PMID: 12773379
- PMCID: PMC156772
- DOI: 10.1093/emboj/cdg277
NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis
Abstract
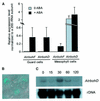
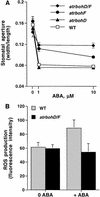
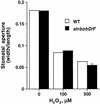
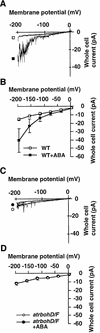
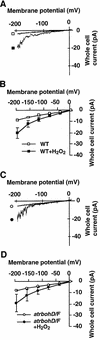
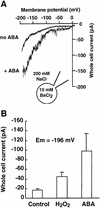
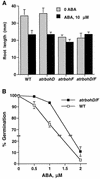
Reactive oxygen species (ROS) have been proposed to function as second messengers in abscisic acid (ABA) signaling in guard cells. However, the question whether ROS production is indeed required for ABA signal transduction in vivo has not yet been addressed, and the molecular mechanisms mediating ROS production during ABA signaling remain unknown. Here, we report identification of two partially redundant Arabidopsis guard cell-expressed NADPH oxidase catalytic subunit genes, AtrbohD and AtrbohF, in which gene disruption impairs ABA signaling. atrbohD/F double mutations impair ABA-induced stomatal closing, ABA promotion of ROS production, ABA-induced cytosolic Ca(2+) increases and ABA- activation of plasma membrane Ca(2+)-permeable channels in guard cells. Exogenous H(2)O(2) rescues both Ca(2+) channel activation and stomatal closing in atrbohD/F. ABA inhibition of seed germination and root elongation are impaired in atrbohD/F, suggesting more general roles for ROS and NADPH oxidases in ABA signaling. These data provide direct molecular genetic and cell biological evidence that ROS are rate-limiting second messengers in ABA signaling, and that the AtrbohD and AtrbohF NADPH oxidases function in guard cell ABA signal transduction.
Figures
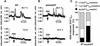
Similar articles
-
AtrbohD and AtrbohF positively regulate abscisic acid-inhibited primary root growth by affecting Ca2+ signalling and auxin response of roots in Arabidopsis.J Exp Bot. 2013 Nov;64(14):4183-92. doi: 10.1093/jxb/ert228. Epub 2013 Aug 20. J Exp Bot. 2013. PMID: 23963673
-
The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production.Plant Physiol. 2007 Mar;143(3):1398-407. doi: 10.1104/pp.106.091298. Epub 2007 Jan 12. Plant Physiol. 2007. PMID: 17220365 Free PMC article.
-
Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells.New Phytol. 2013 Dec;200(4):1049-63. doi: 10.1111/nph.12469. Epub 2013 Sep 3. New Phytol. 2013. PMID: 24033256
-
Signal transduction and ion channels in guard cells.Philos Trans R Soc Lond B Biol Sci. 1998 Sep 29;353(1374):1475-88. doi: 10.1098/rstb.1998.0303. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9800209 Free PMC article. Review.
-
Abscisic acid signaling.Curr Opin Cell Biol. 1995 Apr;7(2):232-8. doi: 10.1016/0955-0674(95)80033-6. Curr Opin Cell Biol. 1995. PMID: 7612276 Review.
Cited by
-
Open or close the gate - stomata action under the control of phytohormones in drought stress conditions.Front Plant Sci. 2013 May 13;4:138. doi: 10.3389/fpls.2013.00138. eCollection 2013. Front Plant Sci. 2013. PMID: 23717320 Free PMC article.
-
FASCICLIN LIKE ARABINOGALACTAN PROTEIN 4 and RESPIRATORY BURST OXIDASE HOMOLOG D and F independently modulate abscisic acid signaling.Plant Signal Behav. 2015;10(2):e989064. doi: 10.4161/15592324.2014.989064. Plant Signal Behav. 2015. PMID: 25826261 Free PMC article.
-
Reactive oxygen species function as signaling molecules in controlling plant development and hormonal responses.Curr Opin Plant Biol. 2022 Oct;69:102293. doi: 10.1016/j.pbi.2022.102293. Epub 2022 Sep 10. Curr Opin Plant Biol. 2022. PMID: 36099672 Free PMC article. Review.
-
Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants.Sci Rep. 2015 Mar 12;5:9018. doi: 10.1038/srep09018. Sci Rep. 2015. PMID: 25761674 Free PMC article.
-
CRISPR/Cas9 edited HSFA6a and HSFA6b of Arabidopsis thaliana offers ABA and osmotic stress insensitivity by modulation of ROS homeostasis.Plant Signal Behav. 2020 Dec 1;15(12):1816321. doi: 10.1080/15592324.2020.1816321. Epub 2020 Sep 16. Plant Signal Behav. 2020. PMID: 32936726 Free PMC article.
References
-
- Allen G.J., Kwak,J.M., Chu,S.P., Llopis,J., Tsien,R.Y., Harper,J.F. and Schroeder,J.I. (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J., 19, 735–747. - PubMed
-
- Allen G.J. et al. (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science, 289, 2338–2342. - PubMed
-
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature, 408, 796–815. - PubMed
-
- Bánfi B., Maturana,A., Jaconi,S., Arnaudeau,S., Laforge,T., Sinha,B., Ligeti,E., Demaurex,N. and Krause,K.-H. (2000) A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science, 287, 138–142. - PubMed
-
- Bokoch G.M. (1994) Regulation of the human neutrophil NADPH oxidase by the Rac GTP-binding proteins. Curr. Opin. Cell Biol., 6, 212–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

