A Domain in the C-terminal region of latency-associated nuclear antigen 1 of Kaposi's sarcoma-associated Herpesvirus affects transcriptional activation and binding to nuclear heterochromatin
- PMID: 12768028
- PMCID: PMC156177
- DOI: 10.1128/jvi.77.12.7093-7100.2003
A Domain in the C-terminal region of latency-associated nuclear antigen 1 of Kaposi's sarcoma-associated Herpesvirus affects transcriptional activation and binding to nuclear heterochromatin
Abstract
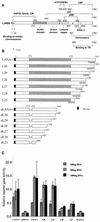
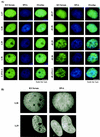
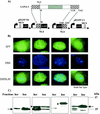
The latency-associated nuclear antigen 1 (LANA-1) of Kaposi's sarcoma-associated herpesvirus (KSHV) is required for the maintenance and replication of viral episomal DNA. The binding sites for nuclear heterochromatin and transcriptional repressor complexes are located in an amino-terminal region of LANA-1, whereas those for viral episomal DNA, p53, pRB, and members of the BRD/fsh family of nuclear proteins are located in its carboxy-terminal domain. LANA-1 activates or represses several cellular and viral promoters. In this report we show that a domain of 15 amino acids (amino acids 1129 to 1143), located close to the carboxy-terminal end of LANA-1, is required for the interaction of LANA-1 with nuclear heterochromatin or nuclear matrix, and for the ability of LANA-1 to activate the Epstein-Barr virus Cp promoter. LANA-1 proteins that are tightly associated with nuclear heterochromatin or matrix differ in molecular weight from LANA-1 proteins that can be dissociated from the nuclear matrix by high-salt buffers, suggesting that posttranslational modifications may determine the association of LANA-1 with nuclear heterochromatin or matrix.
Figures
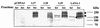
Similar articles
-
The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells.J Virol. 2002 Nov;76(22):11677-87. doi: 10.1128/jvi.76.22.11677-11687.2002. J Virol. 2002. PMID: 12388727 Free PMC article.
-
Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi's sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen.J Virol. 2004 Jul;78(14):7299-310. doi: 10.1128/JVI.78.14.7299-7310.2004. J Virol. 2004. PMID: 15220403 Free PMC article.
-
DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus.J Virol. 2001 Sep;75(17):7882-92. doi: 10.1128/jvi.75.17.7882-7892.2001. J Virol. 2001. PMID: 11483733 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Latency-Associated Nuclear Antigen: Replicating and Shielding Viral DNA during Viral Persistence.J Virol. 2017 Jun 26;91(14):e01083-16. doi: 10.1128/JVI.01083-16. Print 2017 Jul 15. J Virol. 2017. PMID: 28446671 Free PMC article. Review.
-
Structure and function of latency-associated nuclear antigen.Curr Top Microbiol Immunol. 2007;312:101-36. doi: 10.1007/978-3-540-34344-8_4. Curr Top Microbiol Immunol. 2007. PMID: 17089795 Free PMC article. Review.
Cited by
-
Intrabodies targeting the Kaposi sarcoma-associated herpesvirus latency antigen inhibit viral persistence in lymphoma cells.Blood. 2005 Dec 1;106(12):3797-802. doi: 10.1182/blood-2005-04-1627. Epub 2005 Aug 9. Blood. 2005. PMID: 16091453 Free PMC article.
-
Comprehensive analysis of LANA interacting proteins essential for viral genome tethering and persistence.PLoS One. 2013 Sep 11;8(9):e74662. doi: 10.1371/journal.pone.0074662. eCollection 2013. PLoS One. 2013. PMID: 24040311 Free PMC article.
-
Recent advances in the study of Kaposi's sarcoma-associated herpesvirus replication and pathogenesis.Virol Sin. 2015 Apr;30(2):130-45. doi: 10.1007/s12250-015-3595-2. Epub 2015 Apr 23. Virol Sin. 2015. PMID: 25924994 Free PMC article. Review.
-
Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes.J Virol. 2006 Sep;80(18):8909-19. doi: 10.1128/JVI.00502-06. J Virol. 2006. PMID: 16940503 Free PMC article.
-
Phosphorylation of the chromatin binding domain of KSHV LANA.PLoS Pathog. 2012;8(10):e1002972. doi: 10.1371/journal.ppat.1002972. Epub 2012 Oct 18. PLoS Pathog. 2012. PMID: 23093938 Free PMC article.
References
-
- An, J., A. K. Lichtenstein, G. Brent, and M. B. Rettig. 2002. The Kaposi sarcoma-associated herpesvirus (KSHV) induces cellular interleukin 6 expression: role of the KSHV latency-associated nuclear antigen and the AP1 response element. Blood 99:649-654. - PubMed
-
- Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. - PubMed
-
- Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. - PubMed
-
- Boshoff, C., and R. A. Weiss. 1998. Kaposi's sarcoma-associated herpesvirus. Adv. Cancer Res. 75:57-86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

