Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease
- PMID: 12764095
- PMCID: PMC6741113
- DOI: 10.1523/JNEUROSCI.23-10-04081.2003
Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease
Abstract
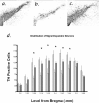
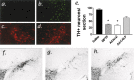
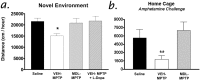
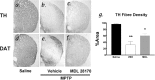
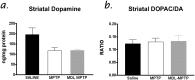
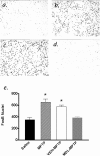
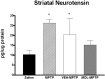
The molecular mechanisms mediating degeneration of midbrain dopamine neurons in Parkinson's disease (PD) are poorly understood. Here, we provide evidence to support a role for the involvement of the calcium-dependent proteases, calpains, in the loss of dopamine neurons in a mouse model of PD. We show that administration of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) evokes an increase in calpain-mediated proteolysis in nigral dopamine neurons in vivo. Inhibition of calpain proteolysis using either a calpain inhibitor (MDL-28170) or adenovirus-mediated overexpression of the endogenous calpain inhibitor protein, calpastatin, significantly attenuated MPTP-induced loss of nigral dopamine neurons. Commensurate with this neuroprotection, MPTP-induced locomotor deficits were abolished, and markers of striatal postsynaptic activity were normalized in calpain inhibitor-treated mice. However, behavioral improvements in MPTP-treated, calpain inhibited mice did not correlate with restored levels of striatal dopamine. These results suggest that protection against nigral neuron degeneration in PD may be sufficient to facilitate normalized locomotor activity without necessitating striatal reinnervation. Immunohistochemical analyses of postmortem midbrain tissues from human PD cases also displayed evidence of increased calpain-related proteolytic activity that was not evident in age-matched control subjects. Taken together, our findings provide a potentially novel correlation between calpain proteolytic activity in an MPTP model of PD and the etiology of neuronal loss in PD in humans.
Figures



Similar articles
-
Downregulation of miR-124 in MPTP-treated mouse model of Parkinson's disease and MPP iodide-treated MN9D cells modulates the expression of the calpain/cdk5 pathway proteins.Neuroscience. 2014 Jul 11;272:167-79. doi: 10.1016/j.neuroscience.2014.04.039. Epub 2014 Apr 30. Neuroscience. 2014. PMID: 24792712
-
Recovery of hypothalamic tuberoinfundibular dopamine neurons from acute toxicant exposure is dependent upon protein synthesis and associated with an increase in parkin and ubiquitin carboxy-terminal hydrolase-L1 expression.Neurotoxicology. 2012 Jun;33(3):321-31. doi: 10.1016/j.neuro.2012.02.001. Epub 2012 Feb 9. Neurotoxicology. 2012. PMID: 22342763 Free PMC article.
-
AAV-Mediated Expression of Dominant-Negative ULK1 Increases Neuronal Survival and Enhances Motor Performance in the MPTP Mouse Model of Parkinson's Disease.Mol Neurobiol. 2020 Feb;57(2):685-697. doi: 10.1007/s12035-019-01744-0. Epub 2019 Aug 24. Mol Neurobiol. 2020. PMID: 31446549
-
The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson's disease.Curr Pharm Des. 2011;17(5):489-507. doi: 10.2174/138161211795164095. Curr Pharm Des. 2011. PMID: 21375482 Review.
-
Calpain as a therapeutic target in traumatic brain injury.Neurotherapeutics. 2010 Jan;7(1):31-42. doi: 10.1016/j.nurt.2009.11.002. Neurotherapeutics. 2010. PMID: 20129495 Free PMC article. Review.
Cited by
-
Absence of glia maturation factor protects dopaminergic neurons and improves motor behavior in mouse model of parkinsonism.Neurochem Res. 2015 May;40(5):980-90. doi: 10.1007/s11064-015-1553-x. Epub 2015 Mar 10. Neurochem Res. 2015. PMID: 25754447 Free PMC article.
-
A Novel Mutation of CAPN1 Gene Causing Hereditary Spastic Paraplegia-76.Ann Indian Acad Neurol. 2022 May-Jun;25(3):555-558. doi: 10.4103/aian.aian_977_21. Epub 2022 May 3. Ann Indian Acad Neurol. 2022. PMID: 35936610 Free PMC article. No abstract available.
-
Paraneoplastic CDR2 and CDR2L antibodies affect Purkinje cell calcium homeostasis.Acta Neuropathol. 2014 Dec;128(6):835-52. doi: 10.1007/s00401-014-1351-6. Epub 2014 Oct 24. Acta Neuropathol. 2014. PMID: 25341622 Free PMC article.
-
Accumulation of α-synuclein in dementia with Lewy bodies is associated with decline in the α-synuclein-degrading enzymes kallikrein-6 and calpain-1.Acta Neuropathol Commun. 2014 Dec 5;2:164. doi: 10.1186/s40478-014-0164-0. Acta Neuropathol Commun. 2014. PMID: 25476568 Free PMC article.
-
UbcH10 overexpression increases carcinogenesis and blocks ALLN susceptibility in colorectal cancer.Sci Rep. 2014 Nov 7;4:6910. doi: 10.1038/srep06910. Sci Rep. 2014. PMID: 25376843 Free PMC article.
References
-
- Abarca J, Gysling K, Roth RH, Bustos G ( 1995) Changes in extracellular levels of glutamate and aspartate in rat substantia nigra induced by dopamine receptor ligands: in vivo microdialysis studies. Neurochem Res 20: 159–169. - PubMed
-
- Abercrombie M ( 1946) Estimation of nuclear population from microtome sections. Anat Rec 94: 239–247. - PubMed
-
- Andersson M, Hilbertson A, Cenci MA ( 1999) Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis 6: 461–474. - PubMed
-
- Beal MF ( 2001) Experimental models of Parkinson's disease. Nat Rev Neurosci 2: 325–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
