In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells
- PMID: 12756227
- PMCID: PMC196056
- DOI: 10.1101/gad.1061803
In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells
Abstract
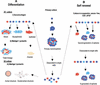
Although the existence of mammary stem cells has been suggested by serial transplantation studies in mice, their identification has been hindered by the lack of specific surface markers, and by the absence of suitable in vitro assays for testing stem cell properties: self-renewal and ability to generate differentiated progeny. We have developed an in vitro cultivation system that allows for propagation of human mammary epithelial cells (HMECs) in an undifferentiated state, based on their ability to proliferate in suspension, as nonadherent mammospheres. We demonstrate that nonadherent mammospheres are enriched in early progenitor/stem cells and able to differentiate along all three mammary epithelial lineages and to clonally generate complex functional structures in reconstituted 3D culture systems. Gene expression analysis of cells isolated from nonadherent mammospheres revealed overlapping genetic programs with other stem and progenitor cells and identified new markers that may be useful in the identification of mammary stem cells. The isolation and characterization of these stem cells should help elucidate the molecular pathways that govern normal mammary development and carcinogenesis.
Figures
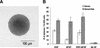




Similar articles
-
A pooled shRNA screen for regulators of primary mammary stem and progenitor cells identifies roles for Asap1 and Prox1.BMC Cancer. 2015 Apr 3;15:221. doi: 10.1186/s12885-015-1187-z. BMC Cancer. 2015. PMID: 25879659 Free PMC article.
-
Stem cells in normal breast development and breast cancer.Cell Prolif. 2003 Oct;36 Suppl 1(Suppl 1):59-72. doi: 10.1046/j.1365-2184.36.s.1.6.x. Cell Prolif. 2003. PMID: 14521516 Free PMC article. Review.
-
Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells.Breast Cancer Res. 2004;6(6):R605-15. doi: 10.1186/bcr920. Epub 2004 Aug 16. Breast Cancer Res. 2004. PMID: 15535842 Free PMC article.
-
Isolation, Culture, and Differentiation of Mammary Epithelial Stem/Progenitor Cells from Fresh or Ex Vivo Cultured Human Breast Tissue.Curr Protoc Cell Biol. 2019 Mar;82(1):e65. doi: 10.1002/cpcb.65. Epub 2018 Sep 28. Curr Protoc Cell Biol. 2019. PMID: 30265440 Free PMC article.
-
Mammary epithelial stem cells.Microsc Res Tech. 2001 Jan 15;52(2):190-203. doi: 10.1002/1097-0029(20010115)52:2<190::AID-JEMT1005>3.0.CO;2-O. Microsc Res Tech. 2001. PMID: 11169867 Review.
Cited by
-
Breast cancer cells promote a notch-dependent mesenchymal phenotype in endothelial cells participating to a pro-tumoral niche.J Transl Med. 2015 Jan 27;13:27. doi: 10.1186/s12967-015-0386-3. J Transl Med. 2015. PMID: 25623554 Free PMC article.
-
Form and function: how estrogen and progesterone regulate the mammary epithelial hierarchy.J Mammary Gland Biol Neoplasia. 2015 Jun;20(1-2):9-25. doi: 10.1007/s10911-015-9337-0. Epub 2015 Jul 19. J Mammary Gland Biol Neoplasia. 2015. PMID: 26188694 Free PMC article. Review.
-
Progesterone-inducible cytokeratin 5-positive cells in luminal breast cancer exhibit progenitor properties.Horm Cancer. 2013 Feb;4(1):36-49. doi: 10.1007/s12672-012-0127-5. Epub 2012 Nov 27. Horm Cancer. 2013. PMID: 23184698 Free PMC article.
-
TGFβ/cyclin D1/Smad-mediated inhibition of BMP4 promotes breast cancer stem cell self-renewal activity.Oncogenesis. 2021 Mar 1;10(3):21. doi: 10.1038/s41389-021-00310-5. Oncogenesis. 2021. PMID: 33649296 Free PMC article.
-
The mevalonate precursor enzyme HMGCS1 is a novel marker and key mediator of cancer stem cell enrichment in luminal and basal models of breast cancer.PLoS One. 2020 Jul 21;15(7):e0236187. doi: 10.1371/journal.pone.0236187. eCollection 2020. PLoS One. 2020. PMID: 32692762 Free PMC article.
References
-
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: Entity or function? Cell. 2001;105:829–841. - PubMed
-
- Bocker W, Moll R, Poremba C, Holland R, Van Diest P, Dervan P, Burger H, Wai D, Ina DR, Brandt B, et al. Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: A new cell biological concept. Lab Invest. 2002;8:737–746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
