Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics
- PMID: 12686696
- PMCID: PMC154359
- DOI: 10.1073/pnas.0737381100
Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics
Abstract
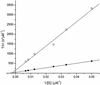
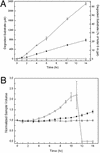
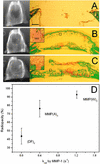
Synthetic hydrogels have been molecularly engineered to mimic the invasive characteristics of native provisional extracellular matrices: a combination of integrin-binding sites and substrates for matrix metalloproteinases (MMP) was required to render the networks degradable and invasive by cells via cell-secreted MMPs. Degradation of gels was engineered starting from a characterization of the degradation kinetics (k(cat) and K(m)) of synthetic MMP substrates in the soluble form and after crosslinking into a 3D hydrogel network. Primary human fibroblasts were demonstrated to proteolytically invade these networks, a process that depended on MMP substrate activity, adhesion ligand concentration, and network crosslinking density. Gels used to deliver recombinant human bone morphogenetic protein-2 to the site of critical defects in rat cranium were completely infiltrated by cells and remodeled into bony tissue within 4 wk at a dose of 5 microg per defect. Bone regeneration was also shown to depend on the proteolytic sensitivity of the matrices. These hydrogels may be useful in tissue engineering and cell biology as alternatives for naturally occurring extracellular matrix-derived materials such as fibrin or collagen.
Figures
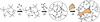

Similar articles
-
In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering.J Biomed Mater Res A. 2010 Dec 1;95(3):673-81. doi: 10.1002/jbm.a.32884. J Biomed Mater Res A. 2010. PMID: 20725983
-
Repair of bone defects using synthetic mimetics of collagenous extracellular matrices.Nat Biotechnol. 2003 May;21(5):513-8. doi: 10.1038/nbt818. Epub 2003 Apr 21. Nat Biotechnol. 2003. PMID: 12704396
-
Synthesis and characterization of matrix metalloprotease sensitive-low molecular weight hyaluronic acid based hydrogels.J Mater Sci Mater Med. 2008 Nov;19(11):3311-8. doi: 10.1007/s10856-008-3469-3. Epub 2008 May 22. J Mater Sci Mater Med. 2008. PMID: 18496734
-
Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part II: biofunctional characteristics.Biomacromolecules. 2006 Nov;7(11):3019-29. doi: 10.1021/bm060504a. Biomacromolecules. 2006. PMID: 17096527
-
Hydrogels as extracellular matrix mimics for 3D cell culture.Biotechnol Bioeng. 2009 Jul 1;103(4):655-63. doi: 10.1002/bit.22361. Biotechnol Bioeng. 2009. PMID: 19472329 Free PMC article. Review.
Cited by
-
A Streamlined High-Throughput LC-MS Assay for Quantifying Peptide Degradation in Cell Culture.bioRxiv [Preprint]. 2024 Oct 15:2024.10.11.617883. doi: 10.1101/2024.10.11.617883. bioRxiv. 2024. Update in: J Biomed Mater Res A. 2025 Jan;113(1):e37864. doi: 10.1002/jbm.a.37864. PMID: 39463983 Free PMC article. Updated. Preprint.
-
Bioresponsive drug delivery systems for the treatment of inflammatory diseases.J Control Release. 2020 Nov 10;327:641-666. doi: 10.1016/j.jconrel.2020.09.008. Epub 2020 Sep 8. J Control Release. 2020. PMID: 32911014 Free PMC article. Review.
-
Characterizing the dynamic rheology in the pericellular region by human mesenchymal stem cell re-engineering in PEG-peptide hydrogel scaffolds.Rheol Acta. 2019 Aug;58(8):421-437. doi: 10.1007/s00397-019-01142-2. Epub 2019 Apr 25. Rheol Acta. 2019. PMID: 32773889 Free PMC article.
-
Harnessing mechanobiology for kidney organoid research.Front Cell Dev Biol. 2023 Nov 24;11:1273923. doi: 10.3389/fcell.2023.1273923. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38077999 Free PMC article. Review.
-
Biodegradable, phosphate-containing, dual-gelling macromers for cellular delivery in bone tissue engineering.Biomaterials. 2015 Oct;67:286-96. doi: 10.1016/j.biomaterials.2015.07.016. Epub 2015 Jul 21. Biomaterials. 2015. PMID: 26232878 Free PMC article.
References
-
- Howe A, Aplin A E, Alahari S K, Juliano R L. Curr Opin Cell Biol. 1998;10:220–231. - PubMed
-
- Schwartz M A, Baron V. Curr Opin Cell Biol. 1999;11:197–202. - PubMed
-
- Streuli C. Curr Opin Cell Biol. 1999;11:634–640. - PubMed
-
- Hubbell J A. Curr Opin Biotechnol. 1999;10:123–129. - PubMed
-
- Griffith L G. Ann NY Acad Sci. 2002;961:83–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

